By: K.S.Trevett and C.M.McKeen (Eurogentec S.A)
The first system tested was a 15-year old design known as the SF-60 and supplied by Genevac Ltd, whilst the second system was a modern computer- controlled evaporation system, the Genevac HT-4. The SF-60has been in regular use for routine drying at Eurogentec for many years, but is known to be very simplisticand to have several drawbacks. Chief amongst these is an inability to control the heat input into the samples, protection from direct heating and lack of temperature control. These problems were entirely solved in the sophisticated design of the HT-4
A variety of oligonucleotide probes were selected for the study, each of which were less than 20 bases in length and were modified at the 5’ and/or 3’ end. Five of each of the following fluorescently labelledoligonucleotides were used :
5’ FAM
5’ Cy5
5’ Cy3
5’ HEX
5’ JOE
5’ TET
5’ FAM 3’ TAMRA
Each of the samples were split equally into three alloquots ; control (which underwent no lyophilisation), and the remaining two which were placed in the HT4 and SF-60 respectively.
The test samples were subsequently evaporated to dryness in their respective instrument. The HT4samples underwent a pre-programmed drying run designed to evaporate water and other solvents, while the SF-60 samples were run until the heat lamp switched off, indicating that all the solvent had evaporated, and thus the run was ended manually.
Following the removal of the probes from the instruments, the oligonucleotides were resuspended in 100µlMilliQ water and vortexed until fully dissolved.
Each of the samples were prepared for mass spectrometry analysis using a Dynamo Maldi-Tof instrumentpowered by a nitrogen laser.
The analyte (3µl) was mixed with cation-exchange resin (3µl), and a hydroxypicolinic acid matrix (3µl), which is necessary for the desorption/ionisation reaction. The analyte-matrix mixture (3µl) was then spotted onto a stainless steel target and allowed to crystallise.
The mass spectra for the Control, HT4 and SF-60 samples were obtained in the positive ion mode, and the acquired molecular weights were compared against the calculated theoretical mass of the test probes toidentify whether the sequence and modification were present and correct.
Following the mass spectrometry analysis, the samples underwent analytical HPLC to verify the presence of the dye and its conjugation to the primer, and to illustrate the sample purity. Each sample (20µg) was suspended in a volume of water (120µl) and filtered before being introduced into a Waters ‘Alliance’separations module coupled to a photodiode array detector. The analytes were subjected to reverse-phasechromatography, with elution facilitated by 95% acetonitrile over a 30 minute gradient.
With the exception of the FAM labelled samples, a significant degradation was observed using the SF60 but no degradation was observed using the HT4. The results of the cy5
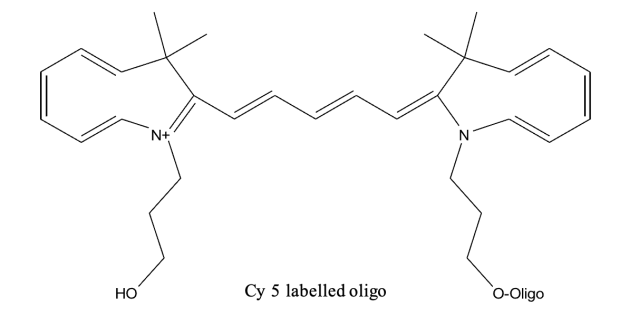
Labelled oligos are shown
The results clearly show that the cy5 has degraded to give a product, while the molecular weight of the product matches the structure below, we have not determined the struction of the degradation product.
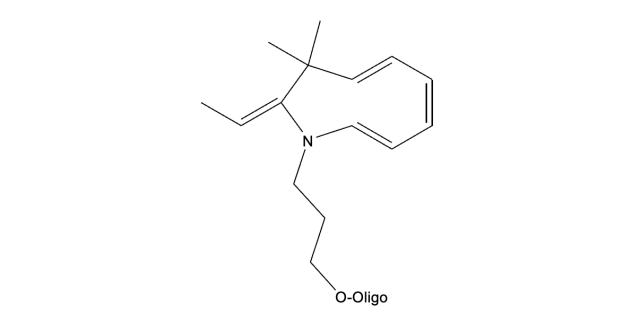
Cy 5 labelled oligo after drying in the SF60
1. (a) Whitcombe D, Theaker J, Guy S P., Brown T, Little, S. Detection of PCR products using self-probingamplicons and fluorescence. Nature Biotechnology (1999), 17, 804-807.
(b) Thelwell N, Millington S, Solinas A, Booth J, Brown T. Mode of action and application of Scorpion primers to mutation detection. Nucleic Acids Res. (2000), 28(19), 3752-61.
(c) Solinas A, Brown LJ, McKeen C, Mellor JM, Nicol J, Thelwell N, Brown T. Duplex Scorpion primers in SNP analysis and FRET applications. Nucleic Acids Res. (2001), 29(20):E96.
2. (a) Solinas, A., Thelwell, N. and Brown, T. Intramolecular TaqMan probes for genetic analysis. Chem.Commun., (2002), 2272-2273
(b) Grove DS. Quantitative real-time polymerase chain reaction for the core facility using TaqMan and the Perkin-Elmer/Applied Biosystems Division 7700 Sequence Detector. Journal of Biomolecular Techniques. (1999), 10, Issue 1, 11-16
3. (a) Bonnet G, Tyagi S, Libchaber A, and Kramer FR. Thermodynamic basis of the enhanced specificity of structured DNA probes. Proc Natl Acad Sci USA (1999), 96, 6171-6176.
(b) Marras SAE, Kramer FR, and Tyagi S Multiplex detection of single- nucleotide variations using molecular beacons. Genet Anal (1999), 14, 151-156.
(c) Marras SAE, Kramer FR, and Tyagi S Efficiencies of fluorescence resonance energy transfer andcontact-mediated quenching in oligonucleotide probes. Nucleic Acids Res (2002), 30, E122
