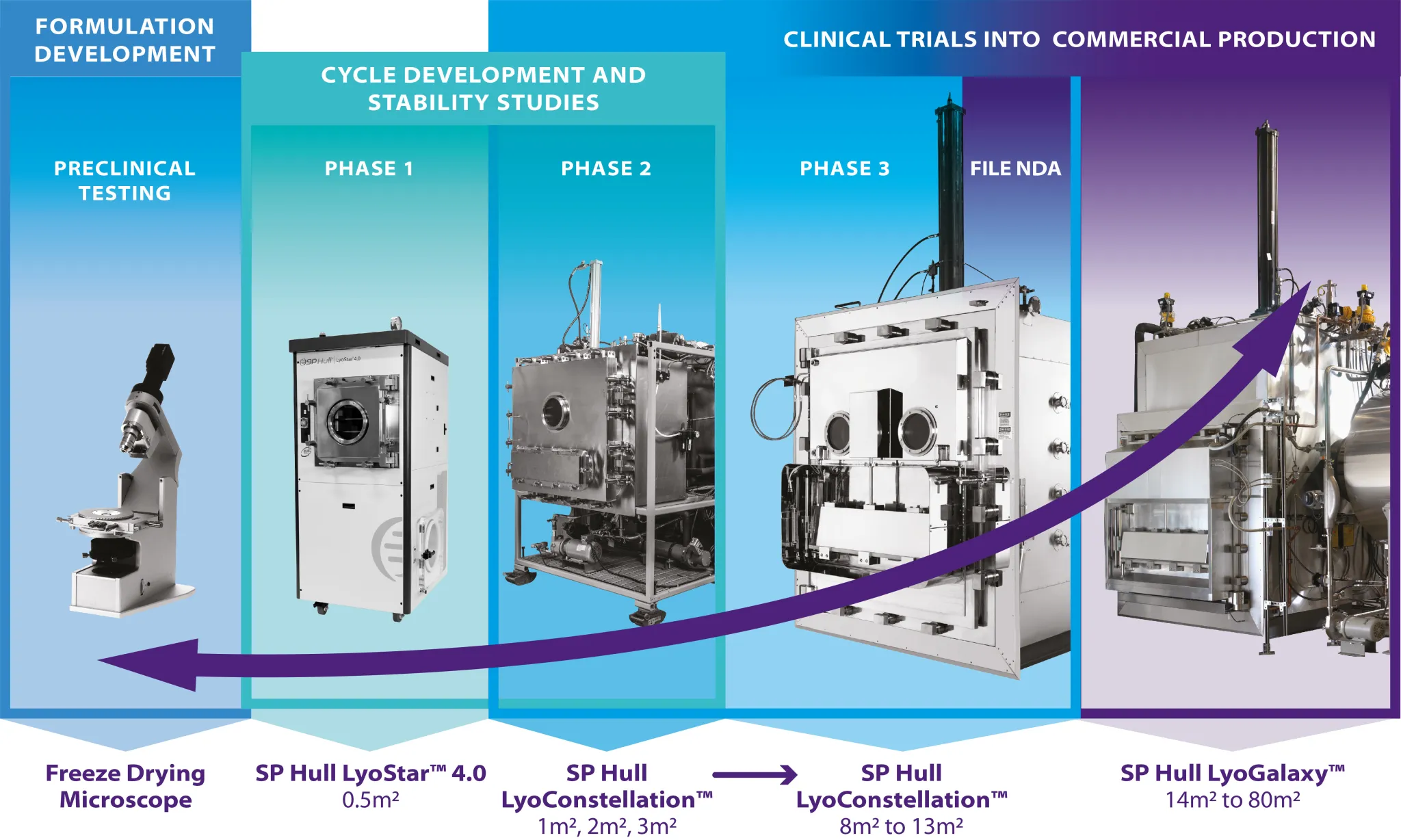
-
Products Pharmaceutical Processing Equipment Fill-Finish / Aseptic Processing Equipment Aseptic & Production-Scale Freeze Dryers Lyophilization Technology & PAT Tools
-
Applications
Find Products by Applications & Industries
- Brands
-
Learning Lab
Explore the Learning Lab
- Service & Support
-
About Us
Learn more about SP


Frequently used links
Product Finder
Product Type
Benchtop Solvent Evaporators
Development, Pilot & Production Freeze Dryers
Fill-Finish / Aseptic Processing Equipment
Laboratory Glassware Washers
Lyophilization Technology and PAT Tools
Research & Pilot Freeze Dryers
Stability Testing
Thermal Management Solutions
Segment/Industries
Aerospace
Agriculture
Analytical Chemistry/BioChemistry
Animal Health
Automotive
Biopsy Labs
Biotechnology
CRO/CDMO
Cartilage & Muscle Research
Chemical Manufacturing
Contract Manufacturing
Cosmetics
Defense
Diagnostic
Differential Scanning Calorimeters
Environmental
Environmental Testing
Food/Beverage
Frozens Labs
General Laboratories
Health Services/Medical
Horse & Cattle Cryolabs
Hospitals
Laboratories
Manufacturing
Medical Devices
Metallurgical Labs
MoHS Labs
Museums
NMR Labs
NMR Manufacturers
Nutraceuticals/Natural Products
Oil and Gas
Petrochemical
Pharmaceutical
Quality Control/Testing
Semiconductor
Steel Manufacturing
Steel Testing Facilities
Tissue
Transgenic Labs
Universities
Universities - Biochemistry
Universities - Chemistry
Universities - Chemistry & Biochemistry
Universities- Embryology & Biology
University - Chemistry & Biology
University/R&D
University/Research and Development
Vacuum Pump Manufacturers
Veterinarians
Water Treatment
Application
Vaccines
Antibody Drug Conjugates (ADCs)
Bulk API
Potent Compounds
Diagnostic Kits
Tissue
Post purification
Oligo synthesis
Reagents
Peptides and proteins
Calorimeters
Rotary Evaporators
Heat Exchanger for Temperature Testing
Lasers
Reaction Vessels
Medicinal chemistry/ PROTACS
crystallagraphy
ADME & DMPK
Vacuum Pump Protection
Vacuum Ovens
Parylene Coating
Temperature Testing Electronics
Temperature Cycling
Fiber Optics
Telecommunications
RF Microwave
Failure Analysis
Metal Testing
General Lab Use
Electropolishing
Medical Devices
Media kitchens
core facilities
clinical labs
glassware washing
Seeds
Artifacts
Books
Peptides
HPLC Fractions
Concentration for analysis
Cleaning validation
Embryo Freezing
Compouind Management
Natural products research
Molecular biology
Clinical
Parylene
Cold Traps
Dewers
Gas Chromatography
Bending Beam Rheometer
Asphalt Testing
Cold Traps Corrosive Protection
Snap Freezing Tissue Samples
Cryostats
Gene & Protein Expression
Histology
Pathology
NMR
X-Ray Diffraction
Electronic Testing
ICH/FDA Stability Testing
Shelf-Life Testing
Acceleration Studies
CryoFreezing
InVivo
Stem Cells
Vacuum Chambers
Cold Plate
Material Testing
Brand
FTS
Genevac
Hotpack
Hull i-Dositecno
Virtis