From R&D to Commercial Manufacturing
By: Ian Whitehall, CMO SP Industries
Nov 4, 2021 7:55 AM
Abstract
Stabilization by lyophilization is attractive to retain a drug product’s potency, structural integrity and homogeneity, all of which are crucial to the success of a pharmaceutical product. Lyophilization begins by lowering the temperature of the product to below its freezing point to form ice crystals (nucleation). However, this process is random and often results in heterogeneity and poor product reconstitution. This article describes various techniques used to control ice nucleation which have been widely used in research and development environments, and demonstrate its use in production manufacturing. It will focus on one of these methods, ControLyo® which uses a pressurization and depressurization technique to provide simultaneous ice nucleation. The advantages of implementing ControLyo will be discussed including its role in creating a smooth transition between lab- and commercial- scale lyophilization.
Introduction
Controlling reproducibility and inter-/intra- batch variability are key objectives of the manufacturing process for all drugs but particularly in the case of parenteral drugs. Lyophilization is part of this process which stabilizes a drug for storage and transport but requires retention of its potency, structural integrity and homogenous quality. To fully control the freeze-drying process, it is important that each stage of lyophilization and the individual products are thoroughly understood and optimized while, at the same time considering future upscaling of product manufacturing from the R&D laboratory to commercial production.
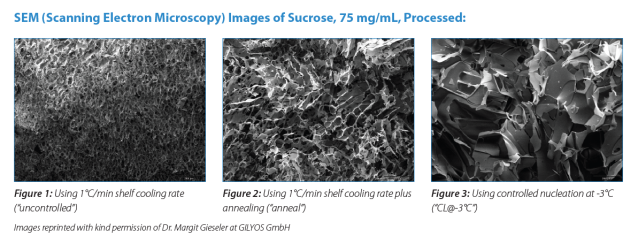
Ice Nucleation
Successful freeze-drying starts with the initiation of the freezing phase (ice nucleation). Ice nucleation is a stochastic (random and uncontrolled) process which makes it challenging to produce a uniform batch of lyophilized products of consistent quality. The contents of individual vials often nucleate over a broad range of temperatures which can span between 10–15°C below the formulation’s thermodynamic freezing point in a laboratory freeze dryer and even greater in a manufacturing cleanroom environment. Vials that nucleate at separate temperatures which will strongly influence the resulting ice crystal size, will dry at various rates and have different pore structure, cake morphology and specific surface area. A high degree of supercooling (colder nucleation temperature) leads to small ice crystals, which in turn results in small pores of high resistance in the product and slower sublimation rates (Figure 1).
To overcome limitations, various methods and technologies have been investigated to initiate nucleation and optimize the drying process. The most common of these include the addition of an annealing cycle or the use of a controlled nucleation technique, such as pressurization and depressurization technique.
Adding an annealing cycle to maintain the formulation above its sub ambient glass transition temperature (Tg’), and below the ice melting temperature for a specific length of time, has been the standard method for many years to create larger ice crystals and minimize the fluctuation in drying rates (Figure 2)[1]. However, this technique can be detrimental to protein stability and doesn’t standardize the nucleation temperature which can still result in batch variability.There are less common alternative approaches to create nucleation, however many of these options are only feasible at the laboratory-scale production of lyophilized samples and are not possible for larger-scale commercial production. Although one of these methods (ice-fog technique) controls nucleation, the vials generally nucleate within a minute or two from each other rather than simultaneously since distribution of the ice-fog in the convoluted freeze dryer chamber can be problematic.
The pressurization/depressurization technique of controlled ice nucleation involves cooling the entire batch of vials to a temperature below the thermodynamic freezing point but above the temperature at which stochastic nucleation starts, followed by pressurization of the freeze-dryer chamber with an inert gas such as nitrogen or argon. When thermal equilibrium, has been achieved, the excess pressure is released rapidly (depressurization), causing ice crystals to form at the top of the solution and propagate throughout the vial within seconds. With this method, ice formation is induced at virtually the same time for all vials in the batch (Figure 3).