By: Dr Induka Abeysena & Rob Darrington
Introduction
For many years lyophilisation, or freeze drying, has been used to dry samples in the laboratory. The technique is well researched and has become the method of choice for many researchers with a few samples to dry. Lyophilisation is often preferred because a high level of dryness is achieved with low residual solvent levels, and because of the light, powdery, ‘fluffy’ finish of the sample which enables the sample to be easily removed and weighted out.
However, there are a number of potential drawbacks encountered with the conventional freezedrying apparatus, these include:
1. Samples must be prepared in a limited range of solvents, normally only water can be used
2. Volatile organic solvents and their mixtures cannot be used
3. The process is slow
Therefore researchers with many samples to process, or mixtures of solvents, say from preparative reverse phase HPLC separation containing water and acetonitrile, have turned to centrifugal evaporators. In other laboratories, compound handling for example, the aggressive nature of the organic solvents used renders a freeze drier unsuitable. Even in for these environments, state of the art centrifugal evaporators, such as the Genevac HT-4X, shown in Figure 1, have some limitations. Problems reported include; samples are dried to a film and therefore may be difficult to resuspend after drying, some samples trap a little residual solvent, and some samples fail to dry with the majority.
Figure 1
Genevac HT-4X Centrifugal Evaporator

In this study we will present the results of research done in the Genevac laboratory to develop a ‘best ofboth worlds’ solution, where rapid parallel drying can be achieved while providing high levels of dryness, the ‘fluffy’ finish desired by many, and where every sample driesevery time.
Problems with Purification
Within purification laboratories samples are typically presented dissolved in water and acetonitrile, with a low level of a modifier present, normally 0.1% TFA. Using a freeze drier to remove these solvents is fraught with difficulties, firstly, the acetonitrile requires a very deep vacuum to freeze it, or a freeze drier which actively freezes the samples. Acetonitrile freezes at –65°C. If the acetonitrile is not frozen thenbumping is inevitable resulting in sample loss and cross contamination.
Secondly, acteonitrile in the ice trap will spoil the vacuum making lyophilisation of the water almost impossible. Thirdly, the process is slow, which is incompatible with the drive to reduce process times within many industries. For these reasons the centrifugal evaporator has become the method of choice, because it issuited to rapidly drying may samples in parallel, and designed to control bumping when drying solventmixtures.
However, there are two potential problems, both are sample related effects. Users report that they experience difficulties with drying a few samples per batch. The problems may be that not all the TFA is removed, residual TFA may damage the sample when in storage, and secondly, the compound may interact with the water boosting the boiling point, making drying difficult. Additionally, residual solvent shows up in Nuclear Magnetic Resonance (NMR) analysis. Whilst these problems occur occasionally, the implications of picking a few samples by hand are prohibitive for many automated laboratories, thereforethe whole sample rack is reprocessed.
Lyophilisation in a Centrifugal Evaporator
Samples prepared in water can be lyophilised in a centrifugal evaporator by pulling the best vacuum available, in the Genevac HT-4X (Figure 1) fitted with the solvent resistant scroll pump, the ultimate vacuum is well below 0.5mbar which is more than adequate to freeze water. This process is akin to normal freeze drying and therefore slow.
Genevac developed a process some years ago where by users can evaporate some of the solvent using all the speed of a centrifugal evaporator, and then switch to a lyophilisation mode when only a few millilitres of solvent are left, thus delivering the best of both worlds. For one Genevac customer this took process time for 96 x 30ml fractions from 48 hours in a freeze drier, to 16 hours (an overnight process) in a Genevac HT-12, equivalent to only 10 minutes per 30ml sample, were the samples dried sequentially. Using this as a platform, the effects of heating a sample during lyophilisation were studied to determine if this gave a speedadvantage.
The study of water containing samples was in two halves. Initially just water was used to develop the optimum conditions, and then with water and acetonitrile to simulate samples taken from HPLC.
Lyophilisation of Water
In all trials Ibuprofen sodium salt was used as the standard sample. A stock solution of 0.01M was prepared in water. 15mls of solution was loaded into each of 48 20ml scintillation vials (Wheaton) and placed dried in a Genevac HT-4X evaporator under various conditions. Figure 2 shows a typical vial holder.
Figure 2
Genevac 20ml scintillation vial holder.

Figure 3 shows a plot of concentration and then lyophilisation of water, this was developed to establish a baseline. The settings used are summarised in Figure 4. The total time taken to dry the sample is approximately 8 hours, 4 hours of concentration in stage 1 & 2, where the sample temperature is at about +8°C and then stage 2, the lyophilisation stage where the sample is frozen to –16°C and warms up whendry.
Figure 3
Baseline method showing concentration and lyophilisation of water based sample – Trial 1
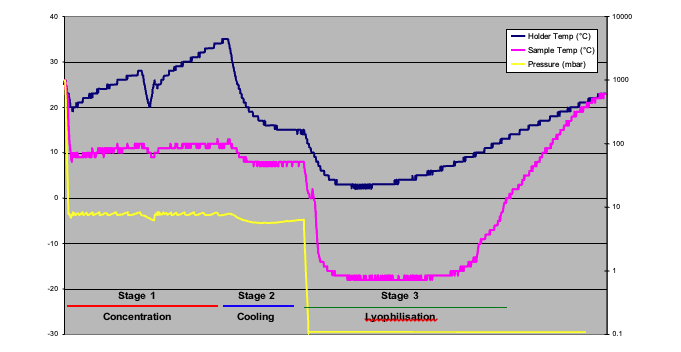
Whilst there are three stages shown in Figure 3, the actual evaporation method comprises up to four stages:
1. Concentration of the bulk of the solvent using fast evaporation
2. Cooling of the samples and sample holders, in preparation for
3. Freezing of the sample using deep vacuum
4. Lyophilising the residual solvent, with or without heat
Figure 4
Results of Lyophilisation trials with water
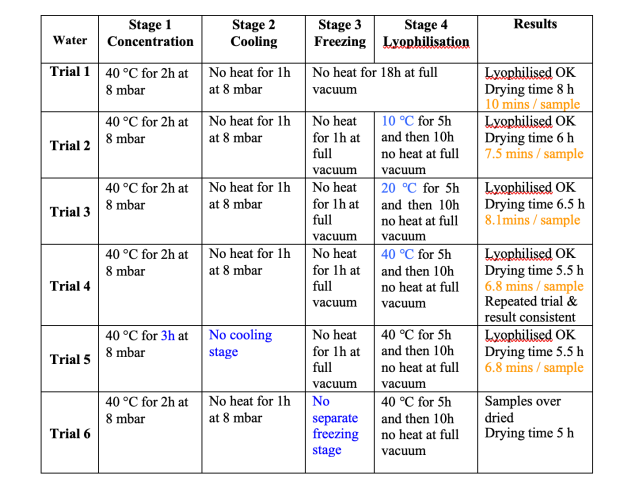
Figure 4 shows the results of the method development for the water processing stage. During sequential runs different heating levels were used in the lyophilisation stage, higher heat levels were shown to reduce the total processing time from 8 hours in trial 1, to 5.5 hours in trial 4. Figure 5 shows the results of trial 4.
The precise processing time for each trial was not known, therefore each sample was over processed, and then from the data the end point was identified. For the sake of uniformity, the time at which the sample temperature became positive was taken as the end point. At this time the samples are warming up rapidly, because there is no longer any cooling effect from lyophilisation.
Figure 5 – Results of concentration and lyophilisation with heating, Trial 4
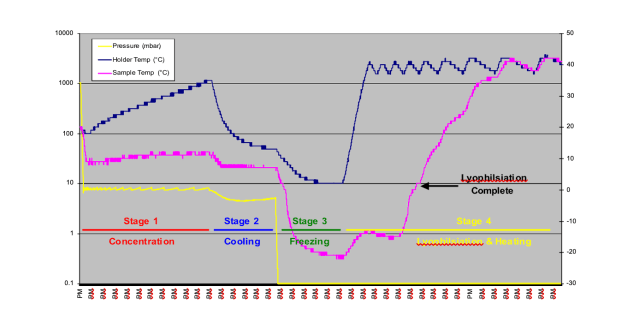
Points to note from these data – we had thought that best practice demanded a cooling stage, stage 2, this had always worked in the past, but had never been tested. In effect the freezing stage achieves cooling as well as freezing, therefore this stage is not necessary, as demonstrated by trial 5. However, the freezing stage with no heat appears to be essential, as shown by trial 6, in this case the samples dried normally, and did not lyophilise, it was evident from the data that the sample had not frozen at all. The results of trial 3 appear to be anomalous, in that higher heat should reduce the processing time, but does not in this trial.
Lyophilisation of Water & Acetonitrile
For many users lyophilisation of water is trivial, whilst the time savings demonstrated in our study are welcome, the issue remains of how to deal with solvent mixtures. A modification to the method used for water is the addition of an earlier stage, stage 0, to remove the acetonitrile before concentration of the water.
Stage 0 has three parts:
1. Dri-PureTM – vacuum ramping and high rotor speed to prevent bumping
2. Concentration – a 40mbar stage to remove the acetonitrile without freezing the water, at 40mbaracetonitrile boils at +2°C
3. Draining the condenser – residual acetonitrile will spoil the vacuum in later stages, therefore must be removed.
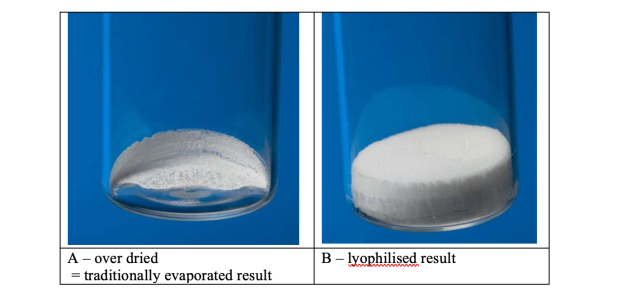
For these trials a 0.01M solution of Ibuprofen sodium salt was prepared in a 60:40 mixture of water and acetonitrile. Figure 6summarises the results. As is evident from the data, it was only at trial 10 that the optimum conditions were achieved. As withthe water trials, the cooling stage was not required. The process needed adjustment to achieve the correct balance of concentration and lyophilisation. 48 x 15ml samples dried in 5 hours, equivalent to 6.25 minutes if the samples were driedsequentially. Figure 7 shows the difference between a lyophilised result, achieved in trial 10, and traditionally centrifugally evaporated sample as per trials 7, 8, 9 and 11. The difference is stark, and the ease of resuspension is greater with the lyophilisedsample, where as the dried sample does not fully dissolve readily. The resuspension of the samples can be viewed at http://www.genevac.com/products/lyophilisation.html
Figure 6
Results of Lyophilisation trials with Water and Acetonitrile
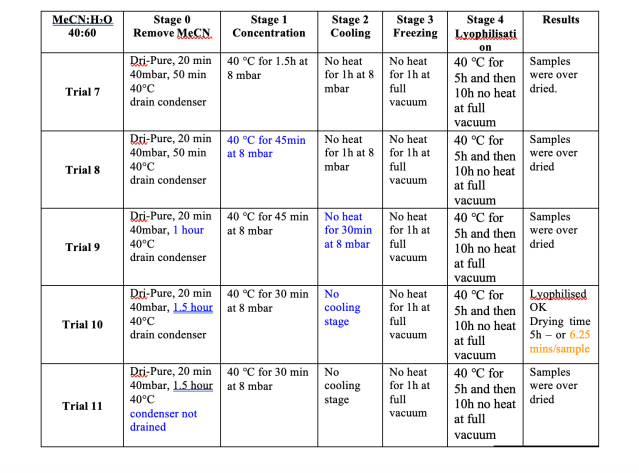
Figure 7
Dried samples in Scintillation vials
Conclusions & Discussion
Lyophilisation of the samples improved the ease of redissolving the sample post drying. The addition of heat during the lyophilisation stage reduced the lyophilisation time considerably. When establishing the lyophilisation method, the traditional cooling stage is not required, but the freezing stage has been shown to be essential.
When evaporating water and acetonitrile mixtures it is necessary to drain the condenser following evaporation of the acetonitrile and before evaporation and lyophilisation of the water. Residual acetonitrile in the condenser spoils the vacuum preventing lyophilisation conditions being achieved.
Using the HT-4X the system had to be drained manually at the end of stage 0, some Genevac systems are able to automate this facility eliminating the need for user intervention mid process. An option to automatethis on the Genevac HT-4X and HT- 12 is being developed as a result of this work.
