By: Nicolas Falquet, Gilles d’Esperonnat & Rob Darrington
Introduction
Polycyclic Aromatic Hydrocarbons (PAHs) are large class of compounds comprising two or more fused aromatic rings. PAHs are naturally occurring in fossil fuels and their derived products and can be formed during incomplete combustion of carbon based fuels. As such they are a by-product of many industrial processes. PAHs vary greatly in size, nature and hazard to human health, some are not classified as toxic, where as others are known carcinogens. The IARC specified 16 as being of particular interest, others have subsequently added this list. In all, over 100 PAHs have been described.
Given the risks and potential risks to human health presented by PAHs, many high risk organisations, such as Foundries, Bitumen Works & Smoke Houses routinely monitor workers and their environment for PAH levels. Typically PAHs are trapped using filters (particulate forms) or resins such as XAD2 (gaseous forms) through which work place environmental air is drawn. Filters may be situated in a small device attached to the workers overalls, or from larger units measuring the air in a wider area. Potential problems exist when recovering the PAHs from the filters and preparing the samples for analysis, principally, losses due to PAH volatility are reported for bi- and tri-cyclic PAHs (ISO11338-2:2003). Therefore, ITGA undertook a study to improve sample recovery and therefore PAH determination when working with low and very low levels of analytes.
Sample Preparation Methodology
Methods for workplace sampling are well described in the literature (NFX43-294 and Method Metropol 011) and result in samples trapped on glass or quartz fibre filters. The filters are preserved and delivered to the analytical laboratory. The whole filter placed into a barcoded vial, 10ml dichloromethane (DCM) is added and the tube placed in an ultrasonic bath at room temperature for 15 minutes to extract the analytes. This operation is repeated once with 10ml of DCM to optimise extraction. Following extraction the sample is concentrated to 1ml using a nitrogen blowing system and then analysed via HPLC coupled to a Fluorescence detector. XAD2 resin tubes may be used as an alternative to fibre filters.

Evaluation of new Sample Preparation Methodology
A standard solution containing the US-EPA 16 PAHs (as defined by IARC, 1987) was spiked onto quartz fibre filters or XAD2 resin tubes and allowed to air dry. The filters / tubes were then extracted twice using 7ml DCM and sonnication in the ultrasonic bath for 15 minutes at room temperature. The combined sample (14ml) had a 100ul aliquot removed. This was made up to 1ml with acetonitrile was taken and injected into HPLC-Fluorescence to provide a 100% reference. The remaining DCM had 100l 2-pentanol added as a solvent keep and was evaporated via centrifugal vacuum evaporation in the Genevac EZ-2 Envi (Figure 1). Temperature and pressure during evaporation were controlled such that the DCM evaporates but the 2-pentanol does not, as previously described by Marsico (2006) and Massat et al. (2007).
Figure 1 (right) – Genevac EZ-2 Envi
The samples were then made up to 1ml using acetonitrile and injected into HPLCFluorescence for analysis. Recoveries for all analytes, even the most volatile were in excess of 90% and the fit of the analytical curve to the reference sample was very good, and shown in figure 2 below.
The samples were then made up to 1ml using acetonitrile and injected into HPLCFluorescence for analysis. Recoveries for all analytes, even the most volatile were in excess of 90% and the fit of the analytical curve to the reference sample was very good, and shown in figure 2 below.
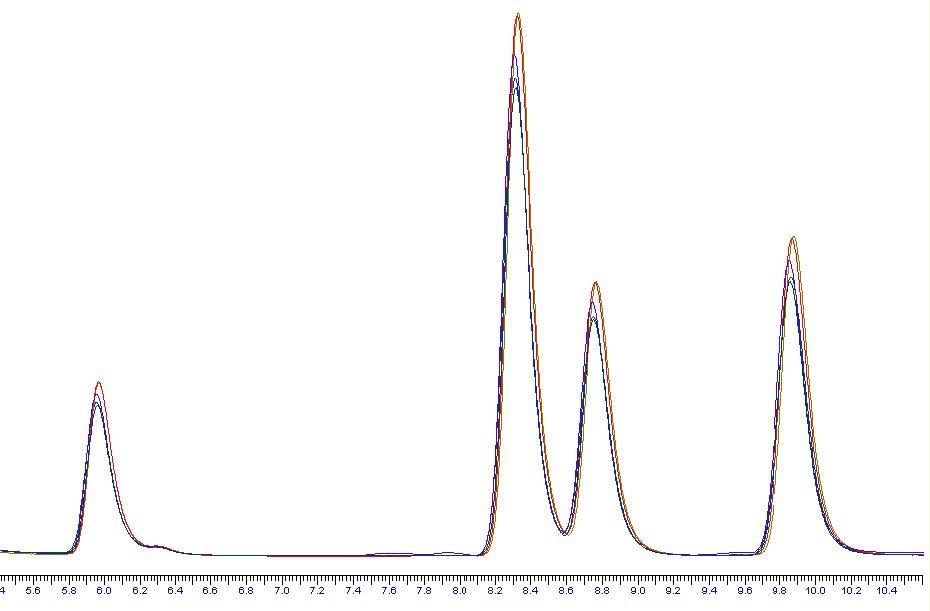
Figure 2 – HPLC-Fluorescence Chromatogram Overlay of Reference Sample to Post Concentration Sample
Red – the reference point. Blue – other chromatograms refer to the PAH compounds Naphthalene, Acenaphthene, Fluorene, Phenanthrene
Validation of the Process
Having delivered similar results to the existing method, and being beneficial in the sense of “automation” of the concentration process, statistical validation of the process and equipment was required. Using the above methodology, a solution containing 14 PAH samples was spiked onto quartz fibre filters and also on to XAD2 resin tubes. Filters were spiked at 100ng and 10ng. These were allowed to dry and extracted, concentrated and analysed. The process was repeated on six distinct occasions using new samples and solutions on each occasion. The results are presented in Figure 3.
Figure 3 – Data from Validation Studies
Mass Recovered (ng) and Recovery % are averages from each of the 6 repetitions performed. SD is the standard deviation across repetitions.
The results generally show excellent recovery and good standard deviation figures. Due to a contamination from XAD2 resin, for two compounds (naphthalene and acenaphtene) limits of quantification have been validated at 50ng instead of 10ng.
Conclusions
The new method of sample preparation was found to be superior to the existing methods. Recoveries are seemingly a little lower for the 10ng studies because this approaches the limit of detection of the analytical method. Following successful validation and external audit by COFRAC (Comité français d’accréditation) the new method and systems have been adopted into routine daily use.
About the Authors
Nicolas Falquet is Testing Manager at ITGA, a leading independent analytical testing laboratory, based at Le polygone, 46 rue de la Télèmatique, 42000 St-Etienne, France. ITGA is part of the Carso Group.
Gilles d’Esperonnat is responsible for sales and service of Genevac evaporators in France and based in the Lyon area Rob Darrington is Product Manager at the Genevac head office, Farthing Road, Ipswich, IP1 5AP, UK.
References
IARC. 1987. IARC Monographs on the evaluation of carcinogenic risks to humans, supplement 7, Overall evaluation of carcinogenicity: an updating of IARC monographs 1-42. Lyon: International Agency for Research on Cancer
Marsico, Anna Maria, 2006. Improving Analysis of Pesticides – a new method development protocol to increase recovery of volatile compounds. First published in Lab Asia, August 2006 & available via http://www.genevac.org/en/ArticleDetail.asp?S=6&V=1&ProductDownload=81
Massat, F, Planel, B & Venezia, A, 2007, Evaluation of Evaporative Sample Preparation Techniques. First published in International Environmental Technology, March/April 2008, pp 36, and also available via http://genevac.org/en/ArticleDetail.asp?S=6&V=1&ProductDownload=134
NF X 43-294. June 1995. Sampling and analysis of polycyclic aromatic hydrocarbons INRS. 2007. Method Metropol 011. Polycyclic Aromatic Hydrocarbons. NF ISO 11338-2. March 2004. Determination of gas and particle-phase polycyclic aromatic hydrocarbons – Part 2 : sample preparation, clean-up and determination.
