By: SP Genevac
What’s So Special About TFA?
TFA (Trifluoroacetic acid) has several properties that make it very important that you take the right precautions when evaporating it, in order to get optimum performance and prevent damage.
Firstly, it’s an acid. This means that if not handled with care, it will corrode components of a system over time. Next, it is quite volatile (with a boiling point / pressure characteristic pretty similar to methanol). In combination with other solvents it can present a quite “bump-prone” mixture. Thirdly, (and perhaps most infamously), it exhibits “creep”. In simple terms, this means that liquid TFA can climb the sides of a vessel to the height that any TFA vapor reaches. What is more, it can carry with it dissolved compounds, which may then end up actually leaving the vessel. Fourthly, and particularly worryingly for users who run into this problem, it can seep through polypropylene (including some brands of 96 well microtiter plates). This is not evidence of a failure to seal the bottom of the well during molding of the plate – it actually goes through the plastic itself, due to it having an extremely low surface tension. TFA in sufficient concentration will also attack silicone (both silicone rubber seals and the special oil in a standard Genevac Cole pump). Finally, it can soften a PTFE coating on an item that is exposed to liquid TFA for any great length of time.
So how can we deal with all this?
It’s quite simple when you know how. All you need to do (for an HT system) is:
- choose a sample vessel it will not leak through
- prevent creep occurring before evaporation begins
- evaporate it without bumping, without forming any condensation anywhere and without any liquid being thrown or sprayed onto the PFTE coated chamber
- ensure that the TFA vapor is always re-captured in the first condenser pot and not sent through the pump in any great quantity
- ensure that when defrosting the condenser the TFA does not creep back into the evaporator
Choose The Right Hardware
Polyprop plates
It has been conclusively demonstrated at a number of user sites that TFA travels through the 96 well polypropylene plates. The simple test to prove this goes as follows:
Load the plates into a suitable Genevac swing (i.e. with a metal base beneath the plate*). Sandwich a piece of filter paper between the base of the plate and the metal swing. Run the machine for a short while (long enough to get up to full rotational speed for 30 minutes) with just TFA in each well of the MTP. If when you open the system up there are 96 telltale marks on the filter paper, this indicates TFA has passed through the bases of the wells. There is no other mechanism by which this pattern could be made on the filter paper.
Experience so far seems to suggest that conical wells that taper to a point are most vulnerable to this effect. To date an official list of good / bad plates has not been compiled though Genevac would welcome the feedback of any users who have experience with particular brands.
Sometimes this effect comes to light in a different way – the user is running the plates in Genevac “open swings” (not advised*) with the lamps shining directly on the base of the plates. When the plates leak, a set of 8 lines can be seen all around the chamber for each level on the rotor (i.e. 3 sets of 8 lines in an HT12). What is actually happening is that liquid TFA is leaking through the wells, flying off at a tangent, hitting the chamber, and (over time) softening the PTFE coating. Though this softening is to some effect temporary, the continuing impingement by drops of liquid TFA at 60 mph (while the coating is soft) starts to damage the chamber coating.
The Right Swings
The effect known as creep results in TFA climbing up the walls of a vessel. This will happen in time if samples are just left in a microtiter plate. Therefore, one of the best things you can do to prevent problems is not to leave samples sitting in plates any longer than you have to. For example, when loading samples including a lot of TFA into an evaporator, make sure to start the run as soon as possible afterwards.
If creep still occurs (i.e. TFA in small quantities climbs out of the wells) there are special swings available from Genevac. These are designed to catch small amounts of TFA as it leaves the plate (or other vessel) and retains it until it turns to vapor, rather than let it be thrown against the chamber wall as liquid droplets. Both the Fast-Stack deep well (used for carrying two plates per rotor position) and the SideBridge swing are available with this modification. Look for “anti-creep” variants in the Accessories Brochure.
Choose the right lamp glass
One effect of splattering TFA (and some compound) onto the walls of the chamber is that you will also find it gets splattered onto the small rectangular windows that separate the infra red lamps from the chamber. There is a potential failure sequence that goes like this:
- TFA and compound gets splattered on the glass.
- Solvent evaporates leaving a dried deposit.
- Deposit gets hot, particularly in center of the lamp beam where intensity is greatest.
- Compound carburizes and turns black.
- Black patches absorb even more lamp heat.
- Heat leaves glass by conduction from hot spots to edges. This means there is a temperature gradient.
- This means that there is differential expansion.
- The glass breaks, causing (at best) downtime or (at worst) an implosion of glass into the chamber.
It is possible to get a different glass arrangement, especially for use in systems suffering from this problem. The system has two different layers of glass. The inner most (facing the chamber) has an extremely low coefficient of thermal expansion. This means that the list above looks more like this:
- Heat leaves glass by conduction from hot spots to edges. This means there is a temperature gradient.
- So what? (**)
This dual glass system is retrofittable to existing evaporators, or can be specified at manufacture time. There is a third option, however:
- TFA and compound gets splattered on the glass
- Solvent evaporates leaving a dried deposit
- Conscientious operator wipes glass clean before next run.
- Problem solved.
Note that the glass can be cleaned with a lint free cloth and some acetone. It is VERY IMPORTANT that you do not press on the glass while doing this. It sits on a sprung seal and pushing against it can push it off the seal and allow dirt to get into the seal.
(**) It is of course good practice to keep the glass clean whatever type you have.
Program Your System Optimally
Prevent Bumping
If you have a mixture of solvents including TFA, you will certainly want to select Dri-Pure™ as an anti-bumping precaution. If TFA is the most volatile of your solvents, and if you have “Variable Dri-Pure™” enabled on your system, you can shorten the vacuum ramp.
(Contact your sales rep for a document explaining how to do this to best effect). If however the TFA is mixed with another, more volatile, solvent (like methylene chloride) then the standard vacuum ramp may be appropriate.
Give the Condenser a Chance
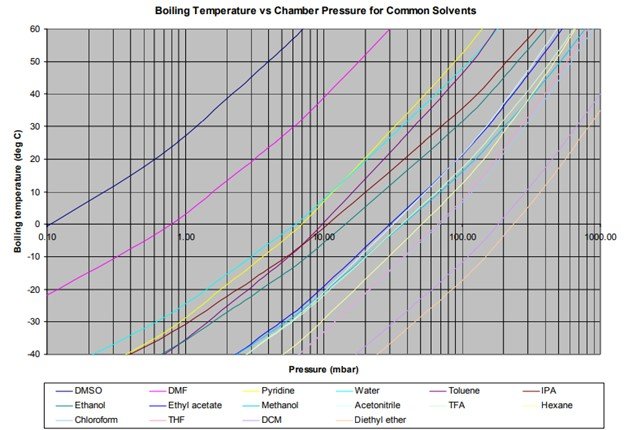
As with all volatile solvents, it is important to choose the correct pressure to optimize the way the condenser deals with the vapor.
For example, if you run TFA with a target pressure of 0.5 mbar, the following happens. At 0.5mbar, the boiling point is –60 deg C. This means it is too cold to condense in the condenser first stage and must go through the pump and be caught in the second stage of the condenser. As vapor is being produced in the evaporator far faster than the pump can pump it out, the pump becomes a serious bottleneck, and a backlog of vapor builds up. This means the target pressure is not achieved, and will probably not go much below 4 or 5 mbar for some time. Since the target is 0.5 mbar, the pump continues pumping in an attempt to lower the pressure, and thus TFA is sent through the pump in great quantity. The pump will suffer over time.
If you instead choose a higher pressure, (12 mbar is a good figure) the boiling point will be –20 deg C, and the condenser will be able to condense the vapor at the same rate at which it is being produced. This means that the target pressure can be achieved, and hence the pump can shut its inlet valve and let the condenser deal with the vapor. This will extend pump life.This means overall that the amount of vapor caught in the first condenser pot is increased, and the amount that travels through the pump to the second pot is decreased.
Of course, there will be some cases where the TFA binds tightly to the compound and so it is impossible to dry the samples completely without dropping the pressure lower than 12mbar. Here’s where some experimentation comes in. Lets say all the TFA that can be removed at 12 mbar is captured in the condenser, and the condenser temperature has dropped back to –45 degrees, but the samples still have some un-evaporated TFA in. It is possible to then drop the pressure to perhaps 4mbar without significant TFA from the first condenser pot migrating through the pump, which may remove the last bits of TFA from the samples. But if you drop the pressure to 2 mbar and below, you will start to see significant amounts of TFA travelling through the pump to the second pot (and beyond).
If dropping this lower is the only way that you can get your samples fully dry, then of course, it has to be done.
Initial Transients
So far the situation we’re describing relates to “steady state” drying once the pressure in the chamber has settled down. But there is more to consider. At the start of a run, there is often a fairly rapid onset of boiling producing a lot of vapor when the working level of vacuum is first approached. At these times, the condenser can be temporarily “overwhelmed”, which means that it cannot condense the vapor at the same rate it is being produced (and so some of that vapor goes through the pump). This situation can be prevented by the use of a gentle vacuum ramp, making the onset of boiling less severe.
Vacuum ramping is not just for preventing bumping!
You will know if the vacuum ramp is sufficiently gentle if the system manages to pretty much achieve its target pressure all the way through the ramp. If not, then the condenser is not condensing everything as it arrives, and so it is going through the pump.
Pump Life
For small quantities of TFA, run at the right pressure, the pump will not suffer unduly. However, if strong solutions (20% and more) are run regularly, there will inevitably be some effect on the pump. The silicone oil in the Genevac Cole pump will degrade in time with exposure to a lot of TFA, and in these circumstances Genevac recommend that the pump be “upgraded” to the “CPC” specification. This simply means that a different (more expensive) oil is used and the pump rotational speed is changed to reflect the different viscosity of the CPC oil.
This upgraded can be carried out in the field to an existing pump.
Caring for your condenser
Perhaps the most obvious bit of advice about the condenser is “remember to empty it”. Some users do forget and though the system still (eventually) dries the samples by pulling the solvent out through the pump, the drying times are very long. Therefore it is only likely to be on longer than necessary overnight runs that an un-emptied condenser goes unnoticed.
Samples will only get dry in these circumstances if the run is programmed for a period way longer than it really needs to be, and that in itself might be said to be bad practice. Even if you have all night to do a run, you should still aim to program the system to run only long enough to get the samples dry, because needless prolonged running ages a system prematurely. A system that does an 8 hour run every night then switches off for 8 hours will live 50% longer than one that runs right through to morning for no reason.
Full condensers often occur not so much because they were not emptied, but because they were not emptied fully. This might be because the condenser was not fully defrosted before draining, or because some sort of residue or sludge has built up which is obstructing the draining process. Though not that common, this is still something you should take steps to prevent.
On a system running mostly TFA (with not much of any other solvent), it is wise to “flush” the condenser periodically to prevent such build-ups. To do the best job of this, use a flushing kit. This is a factory option for an HT-4/DD-4 but can be retro-fitted to external condensers (i.e. HT-8/12 and Megas). It consists principally of a “probe” which reaches down into the condenser pot and is used to spray methanol onto the walls of the pot as part of a semi-automated cleaning process.
A slightly less effective, more time consuming, but still worthwhile method (on a system without a flushing kit) is to evaporate a full “dummy run” of pure methanol. On an HT-8 or 12, this should amount to a litre or more (300 mls on an HT-4) and the run should be carried out at a pressure of at least 12 mbar to ensure the methanol is all condensed in the first condenser pot, where most rinsing is required.
IMPORTANT – How to defrost
This section applies equally to Series 1 and Series 2 systems.
The process of defrosting a condenser involves heating the contents of it until all the ice is melted. On a standard Genevac condenser this means that heat is applied for a
predetermined length of time. With TFA, however, there is a risk that you may end up producing hot liquid TFA in the condenser before the defrost cycle is over. This potentially leads to accelerated creep, meaning that the TFA that you have already trapped in the condenser can make its way back up the tubing into the evaporator chamber, causing quite a mess.
There are two ways to prevent this. On a new system that is specified in advance to be for TFA use, a special “defrost” temperature sensor is fitted by Genevac which ensures that defrost heating stops when the liquid in the condenser rises above a certain temperature. This means the TFA can never get hot enough to cause a problem.
But this is only available as a factory option, not as a retrofit.
For systems without this extra sensor, the best way to defrost with TFA is to open the drain valves before commencing the defrost. This ensures that as soon as liquid TFA is formed, it drains out of the condenser, and there is never a large body of liquid TFA being heated.
This technique should only be used when TFA is present in the condenser, not with other solvents. If you are using other aqueous solutions you will find that ice in the condenser thaws a lot quicker if surrounded by warm water, than if you have a dry lump of ice touching the warmed condenser walls in a few points. Hence you should keep the drain valves shut during defrost when not using TFA.
On an HT-4 and DD-4, it is a bit more awkward to defrost with the drain open. On an HT- 4, open the lid, open the drain valves, then close the lid far enough to see the screen (and hence navigate the defrost menu) but not far enough that the lid re-closes the drain valve.
On a DD-4, lower the lid so that you can get at the defrost button, start the defrost cycle, then leave it fully open again, ensuring the drain valves are not closed by the lid.
Automatic Shutdown
The Genevac series 2 systems have a feature called “Autoshutdown”, which ensures that after a run has finished, the system goes into a “sleep” mode. This is the same state that the system boots up in, and the same state the system goes into when you hit STOP twice from the main menu.
The “sleep” state means that
• The condenser is no longer refrigerating
• The pump switches off (after 30 mins of purging to ensure no vapours remain which could condense in the pump).
People often ask “why not go straight into defrost automatically, as well?” The answer is that your samples are still in the evaporator chamber, and the last thing you want is for solvent vapour to re-evaporate from the condenser and condense on your samples.
Autoshutdown is a “system option” (not a “program option”), which means that if it is enabled, it is enabled for all programs. It cannot be selected on a program by program basis. It can be found by choosing “Options” on the main menu screen and then choosing “options” again. Hitting the enter key toggles its status.
It doesn’t matter (wear wise) that the evaporation is over at midnight, if the system continues to run till 8am when you return, that’s 8 hrs more wear. Autoshutdown therefore makes your system live longer.
Another advantage of Autoshutdown is that if the condenser has been off all night it will have got most of the way back to room temperature, and you might find that it is almost ready to drain without any defrost being required. Of course, you should verify that this is the case before relying on it, because not fully draining a condenser before restarting will (over a few runs) potentially lead to a cumulative problem. To verify if overnight shutdown is thawing your condenser, simply drain it in the morning, then do a defrost cycle and see if anything else comes out. If not, the overnight shutdown was enough to thaw it.
Under normal circumstances Genevac would always recommend that this option be enabled, particularly if the system is run overnight.
The one exception to this rule is with TFA.
You might enable Autoshutdown, then find that you open the door of the evaporator and are met with TFA fumes (or worse, liquid TFA in the chamber) the morning after the run, because of creep from the condenser.
In this case, you may find it preferable to disable Autoshutdown, so that the condenser continues to hold the TFA safely refrigerated in the condenser until you return to the system the next morning.
Look after the Seals
Don’t dispose of TFA in the Arctic.
But seriously, nitrile seals can swell on prolonged contact with TFA. In extreme cases, a swollen door seal will prevent the door from closing and then the evaporator is unusable until the door seal is replaced. If there are any spillages of solvent onto the door seals, (or lid seals on an HT-4/DD-4, or the window seal on an HT-12 door) clean them up with methanol immediately. Ensure all staff are aware of this, and you should not have a problem.
You might even want to consider obtaining a spare door/lid seal just in case, if you frequently suffer spillages.
Finally, some tricks of the Trade
Often evaporation does not go the same when there are compounds present as when you are “practising” using just solvents. For one thing, it is often slower.
Another example is as follows. Whereas you might expect a volatile solvent like TFA to be evaporated before water (in a TFA / water mixture), it can be the case that significant amounts of TFA remain bound to the compound at the end of the evaporation of aqueous TFA.
In some cases the problem can be resolved by using other additional solvents. Toluene is popular for assisting in the removal of TFA, and any experience gleaned in this area from using a rotary evaporator will be relevant when using a Genevac.
Another lesser known fact is that the whereas pure TFA suffers creep, addition of water to the mixture reduces the creep (up to about 40% water by which time it has pretty much stopped completely). So if it is possible to run with an aqueous mixture, this will be better from the point of view of creep.
Surviving TFA – A summary
As we have already seen, correct programming will reduce both the condensation and the amount of TFA that travels through the pump (or travels back into the chamber later). Careful choice of microtitre plates and (if necessary) sample swings will ensure that the chamber is never sprayed with liquid TFA. Defrosting the condenser with the drain valve open and also flushing it on a regular basis will greatly reduce problems. Wiping the lamp window glass clean if anything accumulates on it will prevent build up of hard baked on deposits that could cause the glass to break. And by cleaning up all spillages you can prevent swelling seals that could cause the system to lose vacuum.
But if there’s one overriding thing to remember, it is this.
If you have a problem, contact Genevac.
• If you need assistance setting up the system, email service@scientificproducts.com.
• If you think there is a fault, call the service department.
Don’t suffer in silence.