This webinar will summarize and clarify terms and issues concerning the vacuum integrity testing of freeze dryers. Because the lyophilization process occurs in an evacuated vessel, users of freeze dryers face unique challenges in maintaining the sterility of the product in a system under a vacuum. Among these challenges are the measurement of system “tightness” and the establishment of an inleakage criterion that maintains a reasonable assurance of product sterility. System tightness also is important for laboratory dryers to ensure accurate data required for cycle development. With this in mind, the Vacuum Integrity Test is an important part of any Factory Acceptance Test (FAT), Site Acceptance Test (SAT), and/or Operation Qualification (OQ). Presented by Charles D. Dern, P.E. Project Engineer at SP Scientific Pilot and Production Freeze Dryers
-
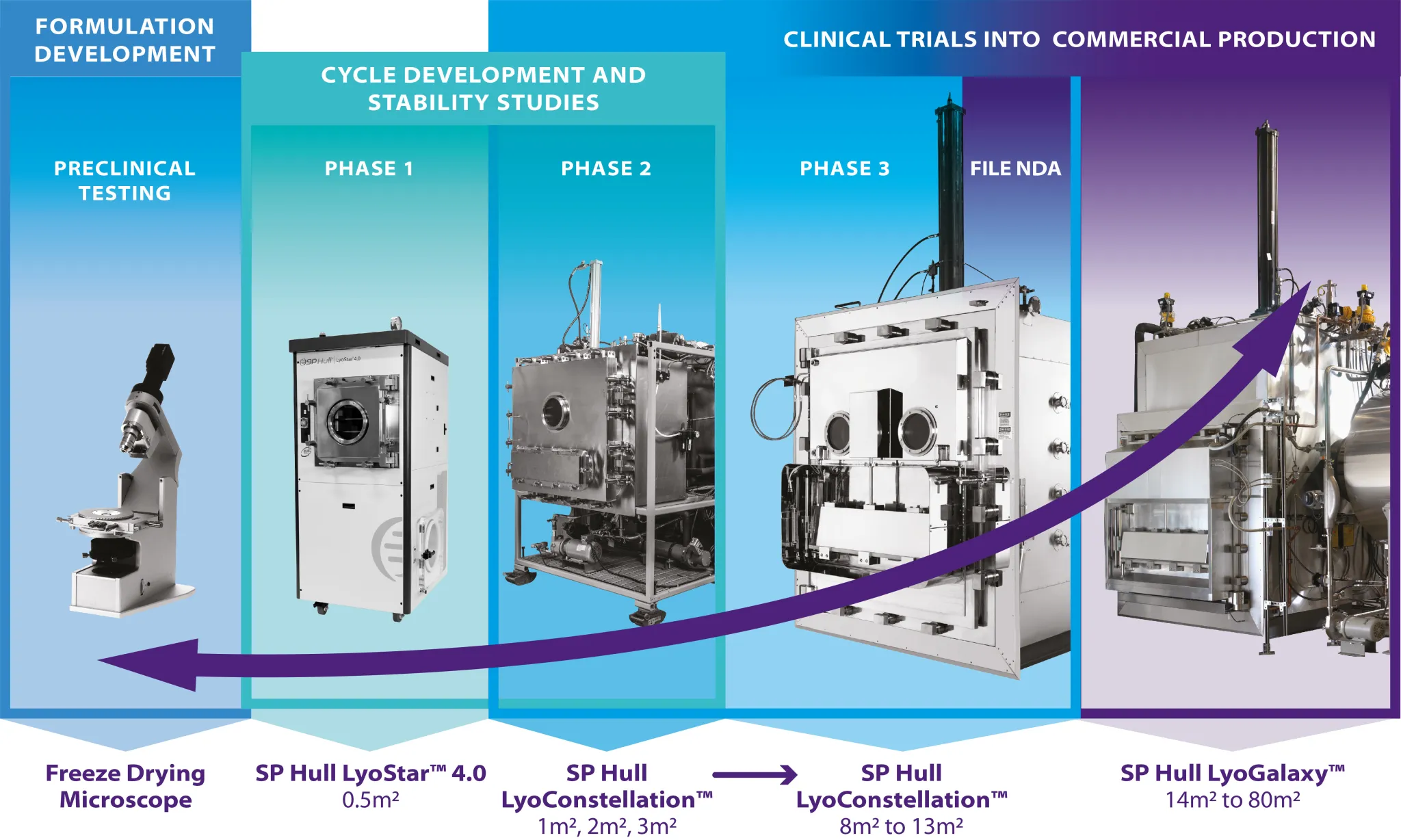
Products Pharmaceutical Processing Equipment Fill-Finish / Aseptic Processing Equipment Aseptic & Production-Scale Freeze Dryers Lyophilization Technology & PAT Tools
-
Applications
Find Products by Applications & Industries
- Brands
-
Learning Lab
Explore the Learning Lab
- Service & Support
-
About Us
Learn more about SP