It is well known that a freeze drying process developed in the laboratory does not necessarily produce the same product temperature history and product quality in manufacturing even though the same shelf temperature, chamber pressure and time settings are identical. Differences arise largely because of differences in ice nucleation temperature, which impacts mass transfer, and heat transfer during primary drying. Historically, scale up has been accomplished largely empirically, with multiple runs and the benefit of prior experience when available. However, in recent years, understanding of these processing differences has improved greatly, and with the use of critical input data and simple heat and mass transfer modeling, scale up can be accomplished much faster and with greater success. This webinar will focus on identification and evaluation of the critical input experimental data and the use of such data to predict intra-batch variations and inter-batch variations, with a focus on scale up. The net effect of all random variations can be evaluate by a simple statistical model for both lab and manufacturing, which then allows prediction of product temperatures and drying times, and therefore prediction of percent of vials dry and percent of vials collapsed and proximity to the edge of failure for a given process. Collapse observations suggest the calculations have useful accuracy. Presented by Dr. Pikal Professor and Distinguished Chair in Pharmaceutical Technology at the University of Connecticut
-
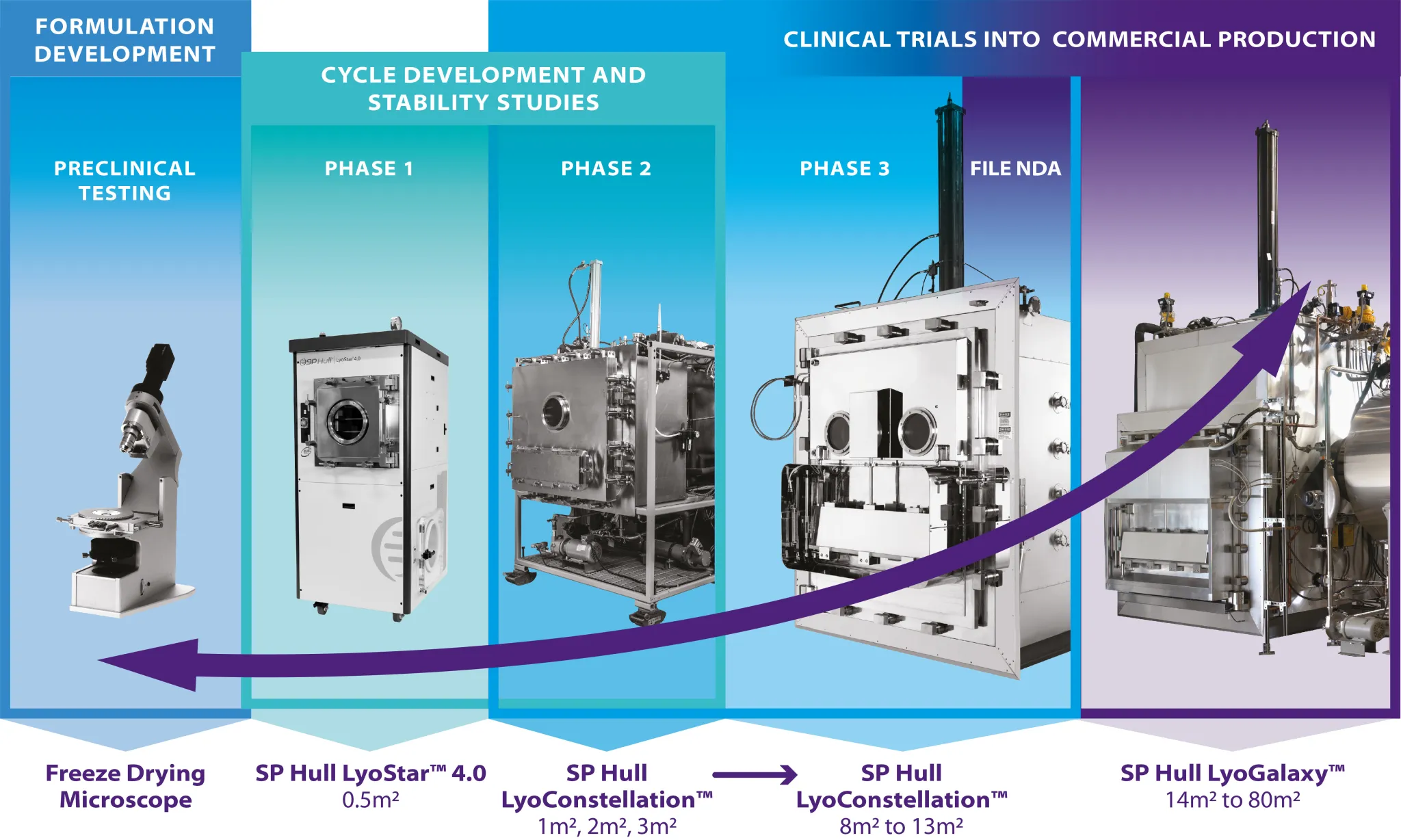
Products Pharmaceutical Processing Equipment Fill-Finish / Aseptic Processing Equipment Aseptic & Production-Scale Freeze Dryers Lyophilization Technology & PAT Tools
-
Applications
Find Products by Applications & Industries
- Brands
-
Learning Lab
Explore the Learning Lab
- Service & Support
-
About Us
Learn more about SP