Reagents typically used in diagnostic test kits for diseases such as COVID-19 tend to contain reactive components such as enzymes or antibodies, which can be challenging to stabilize for commercial use. Lyophilization (freeze-drying) may be considered a relatively gentle drying process, but there are still risks and pitfalls when applying it to such reagents, not least due to freeze-concentration effects and the low volumes of liquid involved. Furthermore, the selection of stabilizers must be made with final assay compatibility in mind, as well as suitability for the lyophilization process itself. This webinar will explore various aspects of formulation and cycle development for diagnostic reagents, including the design/selection of container-closure systems, which can be critical to maintaining activity and stability during transport and storage, especially if the cold chain cannot be maintained throughout distribution. It will also cover the selection of the appropriate lyophilization equipment for a range of product formats/containers, formulations with different critical temperatures, from laboratory research to pilot and large scale production applications. Presented by Dr. Kevin R. Ward, PhD R&D Director at MRSC
-
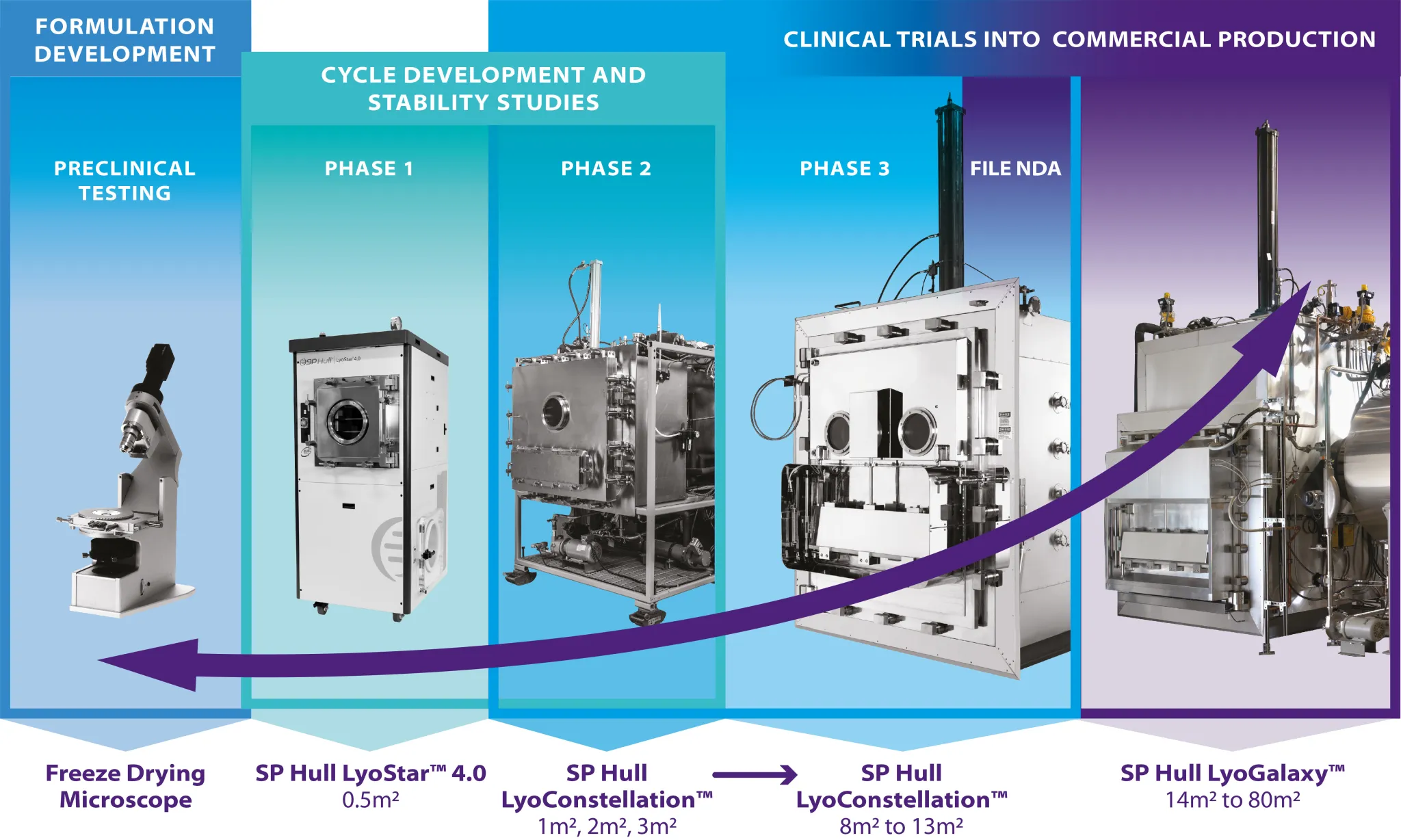
Products Pharmaceutical Processing Equipment Fill-Finish / Aseptic Processing Equipment Aseptic & Production-Scale Freeze Dryers Lyophilization Technology & PAT Tools
-
Applications
Find Products by Applications & Industries
- Brands
-
Learning Lab
Explore the Learning Lab
- Service & Support
-
About Us
Learn more about SP