By: Ngaio Richards, Steve Lancaster, Sarah Hall, Karen Scott
Introduction
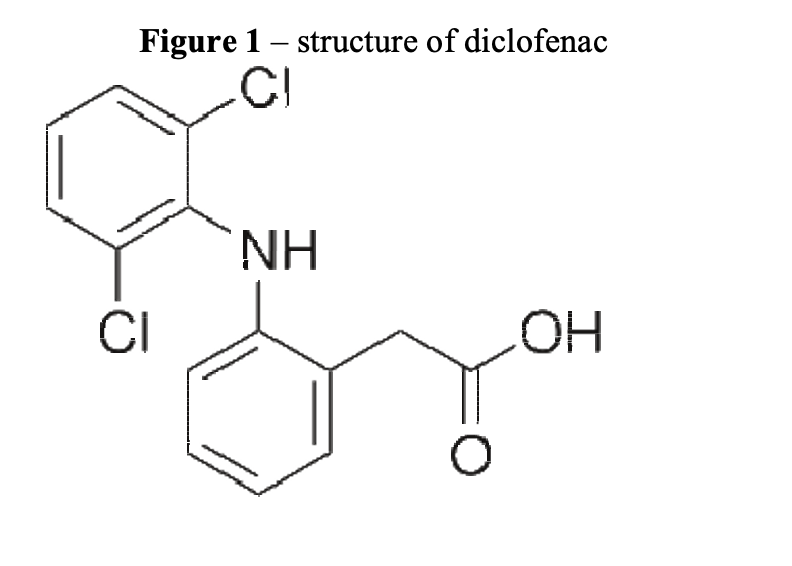
Diclofenac (Figure 1) is a non-steroidal anti- inflammatory drug (NSAID) that is extensively used to treat pain and reduce inflammation in humans and animals. Other commonly encountered NSAIDs include ibuprofen and aspirin. First introduced as an analgesic and anti-inflammatory agent for humans in the 1970s, it was in widespread use in veterinary medicine by the 1980s1.
Diclofenac was registered for veterinary use on the Indian subcontinent in the mid to late 1990s 2,3. Here, livestock animals are often used as working machines, and diclofenac was regarded by veterinarians and farmers as a “wonder drug” because of its evident benefits very quickly after dosage. The drug’s effectiveness, however, arises from its abatement of the symptoms rather than the root cause of the impairment. Multiple administration is often necessary, especially in elderly ailing animals, where nature eventually takes its course!
Traditionally, livestock carcasses on the Indian subcontinent have been left out by the millions for scavengers, particularly vultures, to consume. Over the last decade several species of the vultures that were the primary consumersof these carcasses face extinction, with diclofenac residues in the livestock carcasses implicated in the cause4. Despite the introduction of various regulatory measures to prevent further manufacture of diclofenac within and import intothe Indian subcontinent, the concern remains that stockpiles exist and formulations are still available from nearby countries such as China and Tibet where the manufacture of veterinary diclofenac is legal5. Although clinical trialsidentified the NSAID meloxicam as an effective and safer alternative to diclofenac6,7 it remains more expensive and is therefore not as popular.
The discovery that diclofenac was available for veterinary use in some parts of Africa in 2007 raised concern for those with an interest in the conservation of Africa’s already imperilled vultures and other susceptible avian species. These worries are not confined to diclofenac only. According to a survey of captive facilities; exposure to carprofen, flunixin,ibuprofen, ketoprofen and phenylbutazone may also adversely affect avian species8. Given that NSAIDs are registered worldwide for administration to livestock animals, it is critical to be able to monitor for their presence in the environment, in the carcasses of animals available to any vulnerable species, and in the latter themselves.
Most conventional methods of diclofenac detection still require extraction of the drug residues from the tissues of the dead bird or livestock animal. These samples must be in sufficiently good condition for analysis, therefore retrieval as soon as possible after death is critical. However carcasses, particularly those in remote locations, may not be foundfor days, weeks or months, during which time they can be subjected to extreme environmental conditions. Given these factors, a method which could detect residues in the more long-lived keratinous matrices was deemed useful for long-term monitoring and conservation work.
To address this, an alternate technique was developed to identify diclofenac residues in vultures and livestock animalsby GC-MS (published elsewhere). A preliminary multiscreen method for the simultaneous detection of carprofen, diclofenac, flunixin, ibuprofen, ketoprofen and phenylbutazone was also developed to address the dearth of residue detection methods for the other NSAIDs of concern. The diclofenac detection method was tested using vulture feathers, human hair and nails for eventual application to the analysis of talons, beaks, hooves and bones.
The method was developed principally for dissemination to African laboratories working with Foundation forAnalytical Science & Technology in Africa (FASTA) and particularly to the chemistry laboratory of the Jomo Kenyatta University of Agriculture & Technology (JKUAT) in Nairobi, Kenya. The intention is that the method be routinely used in wildlife forensic investigations both to ascertain the cause of death of African vultures and to evaluate presence or absence of diclofenac and NSAIDs of concern in the agricultural environment. FASTA is a charitable company that was established to support scientific education, analytical research and the preservation of the environment in Africa viacapacity-building and technology transfer.
This paper reviews the development of this methodology, the technical challenges faced, with emphasis on sample preparation, and the role of the miVac sample condenser in increasing the overall efficiency and reliability of the method and its validation.
Development of a method to detect diclofenac in hair, nail and feather samples
The development of the diclofenac detection method required extensive stepwise preliminary trials, namely:assessing its solubility and stability in various solvents, selecting a derivatising agent, optimising derivatisation, establishing instrument sensitivity and testing the extraction process. All sampleswere dried down at 40ºC and reconstituted with derivatising agent before being run on the GC-MS. The methodvalidation comprised extracting samples of hair, nails and feathers in methanol overnight, drying down the extracts and derivatising with N,O-Bis(trimethylsilyl)trifluoroacetamide with 1.0 % trimethylchlorosilane (BSTFA 1.0 % TMCS)and ethyl acetate prior to analysis (Richards 2009, unpublished data).
In the preliminary stages of the research, samples were evaporated to dryness at 40ºC under a steady stream of nitrogen in a Techne Dri-block heater. However, this method was time-consuming, inconvenient and results lacked uniformity. Fitting samples beneath the needles prior to drying could take 10 – 15 minutes. Samples prepared from methanolic solution (1.0 – 2.0 ml) took at least 45 minutes to dry down, frequently over an hour. Extracted samples often tookseveral hours and even then were not completely dry. The system also required close monitoring and samples dried down at different rates within the heater. Reconstitution of samples containing a small residue of methanol resulted in incomplete derivatisation or reactioninversion. Work was occasionally delayed if a shipment of nitrogen was late, and the system was limited to 30 samples at a time.
Sample preparation is the cornerstone of method development. As such it should be easy to carry out, efficient and cost-effective, needing a minimum of consumables, particularly if it will be used in developing countries. Each step must be repeatable and reproducible prior to validation. Rapid and reliable preparation is especially important for anapplication such as this, where mitigating measures and monitoring strategies depend on obtaining accurate results in a timely manner. All samples must be free from artefact so that any incongruous results need not be attributed to sample preparation. Due to the problems outlined above, an alternate method of sample evaporation was required. The miVac DNA concentrator (Genevac Ltd, Ipswich, UK) was selected, not only because it has been successfully used in other similar applications9, but because of the ease and convenience of usage, specifically: the comparative speed of drying, increased number of samples that can be dried down simultaneously, and uniformity of the result. Samples prepared from solution (1.0 and 2.0 ml) required up to 15 minutes only to completely dry down while extracted samples took up to 1 hour. No residual methanol was detected. Up to 44 samples, prepared in either 2.0 or and 4.0 ml vials could bedried down simultaneously. Vial trays can be custom-made to accommodate the preferred vial volume(s). While the trayused for this work was designed to accommodate both 2.0 and
4.0 ml vials, if only 2.0 ml vials had been used then up to 78 samples could have been dried down at one time. Complete dry down was achieved in all case with confidence.
The miVac (Figure 2) dries by boiling the samples under vacuum. As the pressure in the system drops, so does the boiling point and therefore the temperature of the samples. Samples containing methanol will routinely boil at -20°C until they dry when they will warm up to the temperature of the system, typically not more than +40°C. The samplevials are spun in a centrifuge rotor during evaporation to prevent samples boiling over and any resultant sample loss and/or cross contamination. Vial holders can be custom made to accommodate the favoured vial size.
Figure 2 – miVac DNA concentrator

Using the miVac DNA concentrator with solid aluminium rotor, up to 44 samples could be dried down in approximately 15minutes, a great improvement on the nitrogen blow down system. There were no incidents of partial or incomplete drying,eliminating anomalous results due to poor or incomplete derivatisation. In addition, the miVac runs free from operator attention and requires no consumables, rendering suitable for use in areas were the supply chain of scientific materials may be weak.
Conclusion
At least 15,000 samples were dried down over the course of the method development and subsequent validation.Though this may represent a standard weekly run for a commercial laboratory, it is nonetheless a large number for a single PhD researcher to prepare and process. The sample preparation process and method validation were substantially improved by replacing the nitrogen blow down device with the miVac DNA concentrator. The analysis of diclofenac in feathers, hair and nails was facilitated and results could be interpreted with confidence. The concentrator purchased for this research will be shipped to the JKUAT laboratory in Nairobi so that it can be used for this application and in follow-up research.
References
1. Lees, P., Landoni, M.F., Giraudel, J., Toutain, P.L. 2004. Pharmacodynamics and pharmacokinetics of nonsteroidal anti-inflammatory drugs in species of veterinary interest. Journal of Veterinary Pharmacology & Therapeutics, 27, p. 479 – 490.
2. Gilbert, M., Watson, R.T., Virani, M.Z., Oaks, J.L., Ahmed, S., Chaudhry, J.I., Arshad, M., Mahmood, S., Ali, A. & Khan, A.A., 2006. Rapid population declines and mortality clusters in three oriental white-backed vulture (Gyps bengalensis) colonies in Pakistan due to diclofenac poisoning. Oryx, 40, p. 388 – 399.
3. Risebrough, R.W., 2006. Diclofenac: a new environmental poison in south Asia. Journal of the Bombay Natural History Society, 103, p.239 – 250.
4. Oaks, J.L., Gilbert, M., Virani, M.Z., Watson, R.T., Meteyer, C.U., Rideout, B.A., Shivaprasad, H.L., Ahmed, S., Chaudhry, M.J.I., Arshad, M., Mahmood, S., Ali, A. & Khan, A.A., 2004. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature, 427, p. 630- 633.
5. Acharya, R., Cuthbert, R., Sagar Baral, H. & Bahadur Shah, K. 2009. Rapid population declines of Himalayan griffon (Gyps himalayensis) in Upper Mustang, Nepal. Bird Conservation International, 19, p. 99 – 107.
6. Swann, G., Naidoo, V., Cuthbert, R., Green, R.E., Pain, D.J., Swarup, D., Prakash, V., Taggart, M., Bekker, L., Das, D., Diekmann, J.,Diekmann, M., Killian, E., Meharg, A., Patra, R.C., Saini,M. & Wolter, K., 2005. Removing the threat of diclofenac to critically endangered Asian vultures. PLoS Biology, [Online] 31 Jan. 4, p. 395 – 402. Available at: http://biology.plosjournals.org/perlserv/?request=get- document&doi=10.1371/journal.pbio.0040066&ct=1[accessed 21 May 2009].
7. Swarup, D., Patra, R.C., Prakash, V., Cuthbert, R., Das, D., Avari, P., Pain, D.J., Green, R.E., Sharma, A.K., Saini, M. & Taggart, M. 2007, Safety of meloxicam to critically endangered Gyps vultures and other scavenging birds in India, Animal Conservation, 10, p 192 – 198.
8. Cuthbert, R., Parry-Jones, J., Green, R.E. & Pain, D.J., 2006. NSAIDs and scavenging birds: potential impacts beyond Asia’s critically endangered vultures. Biology Letters, [Online] 22 Feb., 3, p. 91 – 94. Available at: http://rsbl.royalsocietypublishing.org/content/3/1/91[accessed 21 May 2009].
9. Goebel, C, 2008, Sample Preparation for the Detection of Synthetic Analogues of Insulin in Human Serum. Laboratory News,January 2008, pp 20.
Acknowledgements
Purchase of the miVac sample condenser was funded by the Foundation for Analytical Science and Technology inAfrica (FASTA).