By: Dr Eleanor I Miller & Dr Simon P Elliott ROAR Forensics, Malvern, UK
Introduction
Hair analysis can be a useful tool in many forensic and clinical applications to establish drug use, trends of use and in the assessment of chronic alcohol consumption. For example, it can be utilised as part of a medico-legal investigation into drug-related deaths (as a complement to testing other post-mortem biological samples), drug-facilitated crimes, as part of programme compliance for those participating in drug or alcohol dependency treatment or as part of workplace or health insurance screening.
Drugs and drug metabolites can become encapsulated within body hair and analysis for these drug residues provides an accurate assessment of an individual’s retrospective drug intake over a period of time (typically months prior to sample collection) delivering more information than an ‘on the spot’ test, e.g. blood or urine, which only offer a snapshot of drug use. A further limitation of a blood or urine sample is that it has to be collected in close proximity to when the drug is taken, or suspected to have been taken, whereas hair for analysis may be collected many weeks later.
Drugs and drug metabolites circulating in the bloodstream pass into the hair follicle and these can become locked into hair strands when they are formed beneath the skin. As the hair
strands grow out, the segment containing any drug metabolites grows with it. It can take several months to grow out in order to allow for an appropriate hair sample collection which is targeting the correct time period under investigation. After this time, testing can potentially determine which drugs were taken and also indicate approximately when they were taken providing evidence of regular, acute or isolated drug use.

Equipment Validation
When introducing any new piece of instrumentation or equipment into a highly controlled environment such as a forensic analysis laboratory, the new unit must be evaluated to ensure that it does not create any artefact in the samples or cross-contaminate the tubes, potentially invalidating the analysis. The Genevac® EZ-2 is a centrifugal vacuum evaporator which can accept many samples, and therefore can be useful in a busy laboratory. With regard to evaporative sample concentration technology the most important issues are prevention of cross contamination and sample recovery, especially for very volatile analytes as some are only present in picogram (pg) quantities.
As part of their evaluation of new equipment, ROAR Forensics evaluated the Genevac EZ-2 before introduction to their processes. A summary of the data is presented in this report, which investigates the potential for cross contamination using a hair alcohol marker and a drugs of abuse (DoA) solution, and, evaluates recovery of amphetamine, which is renowned for its volatility.
Cross Contamination Study
The aim was to determine if any cross-contamination occurred during the evaporation process in the Genevac EZ-2 system for a hair alcohol marker and commonly targeted drugs of abuse in a forensic toxicology hair testing laboratory, at relevant concentrations.
The methodology was based on a previous cross-contamination study involving 96 well micro-titre plates which found that the sample travel was always observed to be horizontal1.
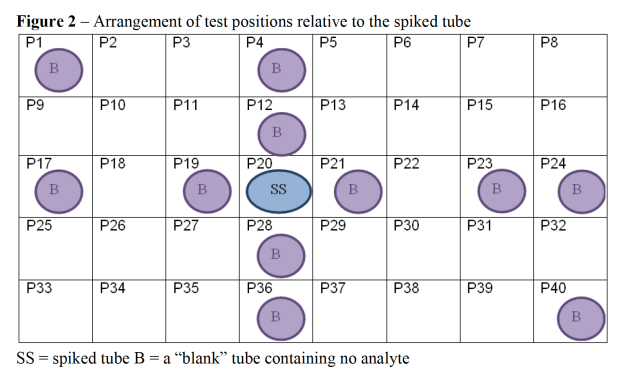
“Blank” tubes containing no analytes were positioned in the sample holder along the row containing the spiked sample, in some adjacent positions and also in a few positions at the furthest points from the spiked sample position. 13mm diameter x 100mm height tubes were used in a Genevac 10-5002 sample holder. The arrangement is shown in Figure 2.
Trials were carried out as follows:
1. 1500pg of a hair alcohol marker – ethylglucuronide (EtG) in 2ml of solid phase extraction (SPE) eluent, a mixture of methanol and formic acid. Two identical sample holders were evaporated in the EZ-2 using method 2, “Low BP”, with the sample holder temperature set to 40°C.
2. 1000ng of a DoA standard solution in 7ml of SPE eluent, a mixture of acetone, dichloromethane, ethyl acetate, and ammonium hydroxide. Two identical sample holders were evaporated in the EZ-2 using method 5, “Low BP Mix” with the sample holder temperature set to 40°C.
The DoA solution contained; ecgonine methyl ester, cocaine, benzoylecgonine, norcocaine, cocaethylene, morphine, 6-monoacetylmorphine, codeine, dihydrocodeine, methadone, EDDP, amphetamine, methamphetamine, MDA, MDMA, MDEA and MBDB.
The levels of analytes were selected because they are the concentrations that produce the highest hair calibrator in each method; 50pg/mg equivalent for EtG and 50ng/mg equivalent for DoA. After evaporation the tubes were reconstituted with 100l of mobile phase and analysed via LC-MS-MS.
Cross Contamination Study Results
No EtG or DOA analytes were detected from analysis of any of the tube contents which were evaporated in positions P1, P4, P12, P17, P19, P21, P23, P24, P28, P36 and P40. Position P20 (“positive” control) showed expected analytes having been spiked with 1500pg EtG or
1000ng DOA.
Analyte Recovery Study
Amphetamine was used for this study due to its renowned volatility. The concentration selected for assessment is the equivalent of 0.2 ng/mg (the current proposed Society of Hair Testing (SoHT) cut-off for an indication of active amphetamine use)2. 4ng of amphetamine in methanol was pipetted into 13 x 100 mm test tubes. Three tubes were evaporated in the EZ-2 using method 5 “Low BP Mix” with the sample holder temperature set at 40°C. Three tubes were allowed sufficient time to evaporate in a fumehood at room temperature. After evaporation the tubes were reconstituted with 100l of mobile phase and analysed via LCMS- MS.
The peak areas for amphetamine were compared for the two different sets of evaporation conditions.
The percentage relative recovery was calculated using the equation below:
% RRecovery = average peak area for amphetamine evaporated using Genevac EZ-2 x 100 average peak area for amphetamine evaporated at room temperature
The recovery for the tubes evaporated in the Genevac EZ-2 relative to the tubes evaporated at room temperature was calculated to be 115 %.
Conclusions
No cross-contamination was observed during the evaporation processes selected for EtG or DOA for the concentrations tested.
Based on the limited data, it would appear that the evaporation system is suitable for evaporating the SPE eluent containing amphetamine, with no loss observed. It would also appear that the Genevac evaporation programme used for DOA produces excellent recovery for amphetamine, which is renowned for its volatility. From an interpretative perspective, it would appear that samples containing amphetamine at the SoHT recommended cut-off of 0.2 ng/mg would be determined at this level.
References
1. Dri-Pure Sample Integrity Protection System. An Evaluation by GlaxoWellcome. Dr Martin Deal (1999), available via www.genevac.com
2. Society of Hair Testing. Recommendations for hair testing in forensic cases. Forensic.Sci.Int. 145:83-84 (2004).
Acknowledgements
The authors would like to acknowledge Eleanor Menzies of King’s College London for her contribution to this evaluation study.
About the Authors
Dr Eleanor I Miller is a Specialist Forensic Toxicologist & Dr Simon P Elliott is Managing Director, at ROAR Forensics, Malvern Hills Science Park, Geraldine Road, Malvern, WR14 3SZ, UK. (www.roarforensics.com)