By: A. Kaufmann; K. Maden and S. Walker
Introduction
Nitrofuran antibiotics were banned from use in the European Union [EU] in 1995 due to concerns that their residues were carcinogenic. In 2002/3 the EU introduced a stringent testing regimen which calls for the use of highly sensitive methods to test food stuffs, principally meat, fish & shellfish, for the presence of this class of antibiotics. The Minimum Required Performance Limit [MRPL] laid down by the EU directive is 1g per kg for edible tissues, and is enforced on all products whether produced locally or imported into the EU. Many papers detail methods and identify metabolites and derivatives of the drugs concerned and are listed by Vass,
Hruska & Franek (2008). The analytical method calls for good upstream sample preparation to eliminate the effects of the matrix, and can be manual and time consuming, particularly where evaporation is concerned. This article describes operational benefits including workflow improvements gained by the official food control authority of the canton of Zurich (Kantonales Labor Zurich) or KLZH during improvement of their upstream sample preparation methodology.
Sample Preparation Methodology
Of the methods cited in the literature for upstream sample preparation, many laboratories favour physico-chemical assays with chromatographic separation and mass spectrometry [MS] detection of metabolites.
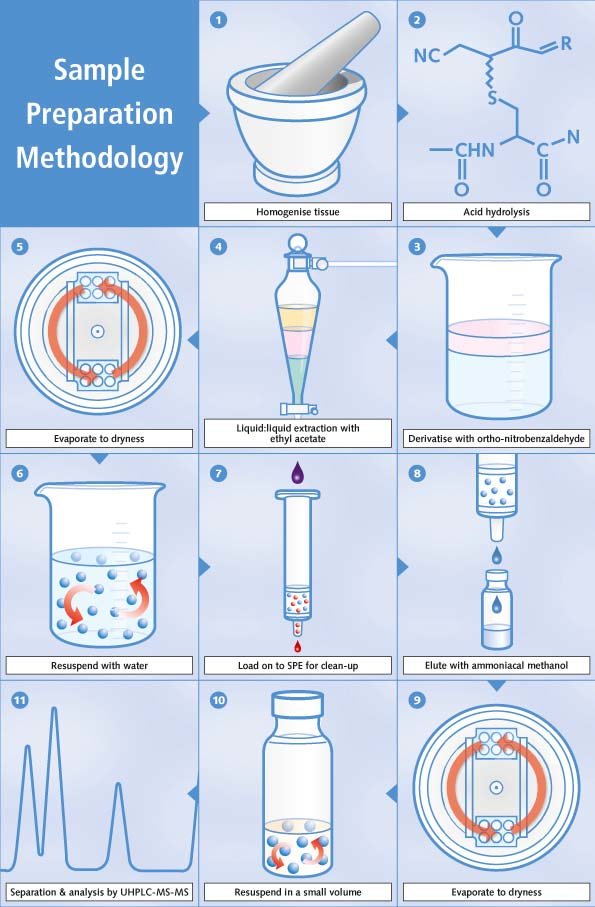
The general scheme for preparation and analysis of nitrofurans in foodstuffs at KLZH is as follows, and summarised in figure 1:
1. Homogenisation of tissue sample
2. Acid hydrolysis to release tissue bound metabolites
3. Derivatisation of metabolites with orthonitrobenzaldehyde to increase molar mass and increase sensitivity of detection
4. Liquid:liquid extaction with ethyl acetate.
5. Evaporation to dryness.
6. Resuspension with water.
7. Clean-up with Solid Phase Extraction to eliminate interfering matrix elements, such as lipids
8. Elution with ammoniacal methanol.
9. Evaporation to dryness
10. Resuspension in a known small volume of solvent suitable for chromatography.
11. Separation via ultra high performance liquid chromatography [UHPLC] and analysis with MS/MS.
The most time consuming and labour intensive parts of this methodology are the extraction and the two evaporation-to-dryness steps.
Traditional Evaporation Technique
At KLZH the evaporation part of the sample preparation method has traditionally relied upon use of a rotary evaporator. The rotary evaporator method for Nitrofuran testing was proven to give good recoveries. However it has a number of operational drawbacks. The biggest disadvantage was that the rotary evaporator is a single sample system which requires continuous monitoring to control the process and to ensure that no foaming or bumping occurs. Samples for Nitrofuran typically presented to KLZH in batches of 5 to 30. Where a small batch is managed in an assessable amount of time with a rotary evaporator, bigger batches would soon become impossible to process. Therefore, three batch process evaporators were evaluated in order to improve the productivity of this step.
New Evaporative Technologies Evaluated
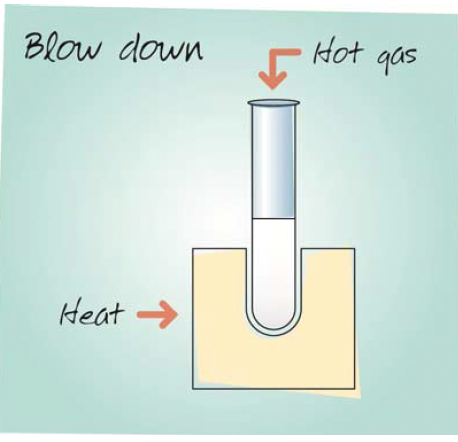
Blow-Down Evaporation
In these evaporator systems, an inert gas such as nitrogen is blown down through needles onto the samples in tubes to create a flow over the liquid surface. This alters the equilibrium between the vapour and liquid phases to favour the vapour phase. Heat is normally applied to the samples to hasten evaporation. Therefore the samples are hot during the process, being at the temperature of the heating block or bath, and consequently the technique offers poor recovery of volatile analytes. Although blow down evaporation is relatively fast for volatile solvents, it can be slow for solvents with high boiling points or those that are difficult to evaporate such as water. Blow down evaporation requires continuous monitoring by the user to detect the end point of the drying process and to optimise the position of the gas jets, keeping them close to the liquid surface.
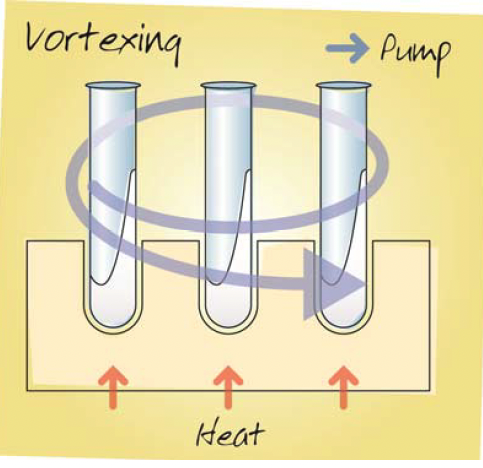
Vortex Evaporation
These systems boil batches of samples under vacuum and therefore the samples are cold throughout evaporation, while swirling the sample tubes to create a vortex. The vortex created generates a large sample surface area for evaporation, making the process relatively fast.
However, the resultant dried product is spread across the vessel walls, which can make sample recovery more difficult. Moreover, in contrast to centrifugal concentrators, the swirling movement generates insufficient g force to prevent solvent bumping and tends to aid foam formation. Hence vortex evaporators are prone to sample loss and cross contamination. Controls on the system can permit hands free operation once the vigour of the vortexing action has been correctly set.
Centrifugal Vacuum Evaporation
Centrifugal evaporators induce solvent boiling under vacuum and so the samples are cold. Centrifugal evaporators use cold traps to recover the vaporised solvent. Centrifugation ensures that solvent boils from the sample surface downwards, thereby eliminating boiling over, foaming and solvent bumping and so preventing sample loss and cross-contamination. Solvent at the liquid surface is at the pressure of the equipment, whereas solvent below this level is at a higher pressure due to the extra weight of solvent multiplied by the g force exerted by the centrifuge rotor. Systems with very high rotor speeds generating 500g or more are proven to prevent solvent bumping. The centrifugal evaporation technique accommodates a wide range of solvents and can concentrate, dry to a film or freeze dry samples. Controls on the system permit hands free operation, with the most advanced systems having automatic detection for the end of the method built in.
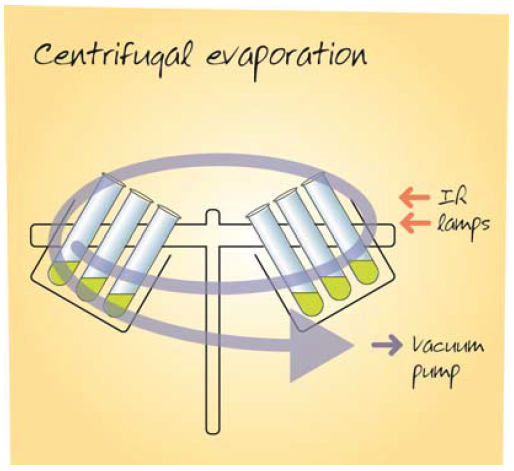
Results of Evaluation
The systems were tested for their suitability for use in the sample preparation process, with particular attention to cross-contamination / bumping / foaming, solvent recovery and degree of user intervention. The results are shown below in Figure 2.
Figure 2 (Below) – Results of solvent evaporation system evaluation
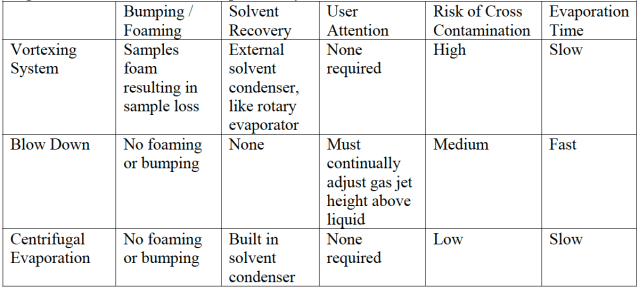
Conclusions
Working with the rotary evaporator, a batch consisting of a total of 20 samples (reference solutions, samples and spikes) would require approximately 1.5 hours (evaporation time) for the first evaporation step and another 2.5 – 3 hours for the second evaporation. A total of 4 – 4.5 hours spent where the operator has to keep an eye on the evaporator and change the samples every few minutes.

The centrifugal evaporator would require for the same batch up to 3 hours for both evaporation steps. In that time, the operator is free to do other work.
The centrifugal evaporator can take a total of 48 nitrofuran samples. These 48 samples would require approximately one hour more to evaporate (both steps) than a batch of 20.
This would be an estimated 2 – 3 hours saved in comparison to working with a rotary evaporator.
Overall the installation of the EZ-2 Envi evaporator (Genevac Ltd, Ipswich, UK. Shown in figure 3) at KLZH is estimated to save the laboratory 2-3 hours per day that previously was spent on evaporation tasks.
The instrument enabled the processing of larger sample series within a given day. It significantly reduced the contamination (carry-over) issue. Previously, the frequent false positive findings were the reason for re-analysis of affected sample or whole sample series.
Very important is also the fact that the EZ-2 Envi operates unattended in a fully automatic manner.
References
Vass, Hruska & Franek. 2008. Nitrofuran Antibiotics: a review on the application, prohibition and residual analysis. Veterinarni Medicina, 53, 2008 (9), pp 269-500.
About the Authors
A. Kaufmann, K. Maden und S. Walker are working at the official food control authority of the canton of Zurich (Kantonales Labor Zurich). The group focuses on the analysis of veterinary drug residues in animal based food products. Commonly uses analytical techniques are Liquid chromatography coupled to high resolution mass spectrometry and liquid chromatography coupled to tandem quadrupole mass spectrometry.