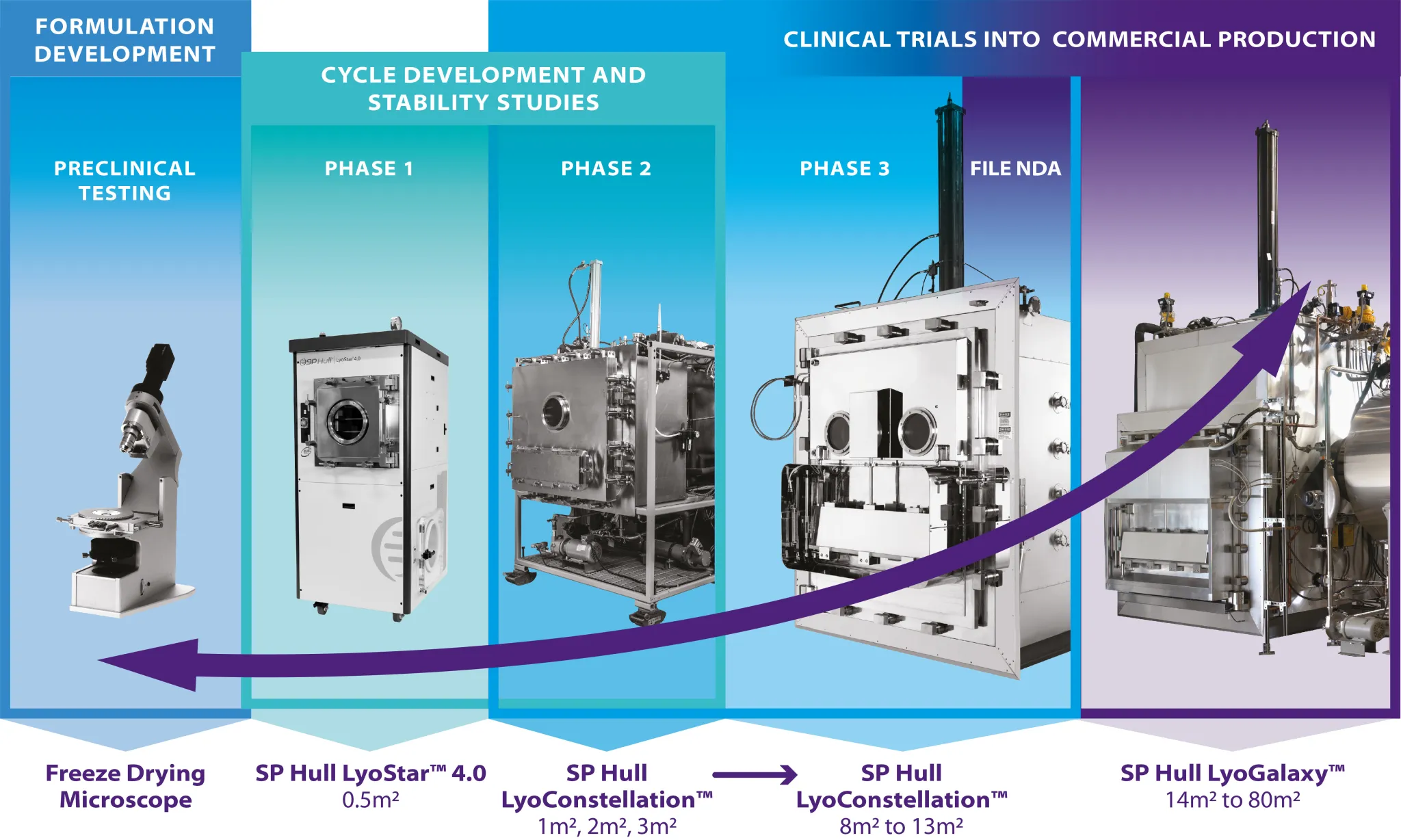
Biopharmaceuticals are routinely freeze-dried to improved product stability and, thereby, achieve acceptable commercial shelf life. However, freeze-drying is a unit operation coupled in formulation and process. While selection of formulation is primarily focused on improving product stability, for a freeze-dried product an additional consideration is the compatibility of the formulation with the freeze-drying process. This presentation will lay out the significance of systematic formulation and process characterization for successfully designing and developing a robust freeze-drying process with acceptable product quality attributes. Elements of formulation, process and drug product presentation (fill volume and recon volume) will be discussed from an industry view point. Presented by Dr. Sajal M. Patel Senior Scientist, Dept. of Formulation Sciences at MedImmune, LLC
-
Products Pharmaceutical Processing Equipment Fill-Finish / Aseptic Processing Equipment Development, Pilot & Production Freeze Dryers Lyophilization Technology & PAT Tools
-
Applications
Find Products by Applications & Industries
- Brands
-
Learning Lab
Explore the Learning Lab
- Service & Support
-
About Us
Learn more about SP