The December LyoLearn Webinar presented by Flow Sciences Inc. in cooperation with Nektar Therapeutics, and SafeBridge Consultants, Inc.,will focus on nanogram level containment of active pharmaceutical ingredients (APIs) for lyophilizers utilizing vented enclosures and gloveboxes. Factory Acceptance Test and Site Acceptance Test data will be explored in particular for several freeze dryer products. A case study examining criteria from client specification of containment levels, consultant interaction and resulting 3-D computer aided design (CAD), computational fluid dynamics (CFD), lean manufacture, and industrial hygiene and assessment certification processes will be discussed. Presented by Allan Goodman, Ph.D.; Lab Manager, Flow Sciences, Inc.; Allan W. Ader, Ph.D., DABT, SafeBridge Consultants Inc.; Richard Hirsh, Director of EH&S for Nektar Therapeutics Q&A Panel: Steve Janz, VP International Sales & Business Development, Flow Sciences, Inc.
-
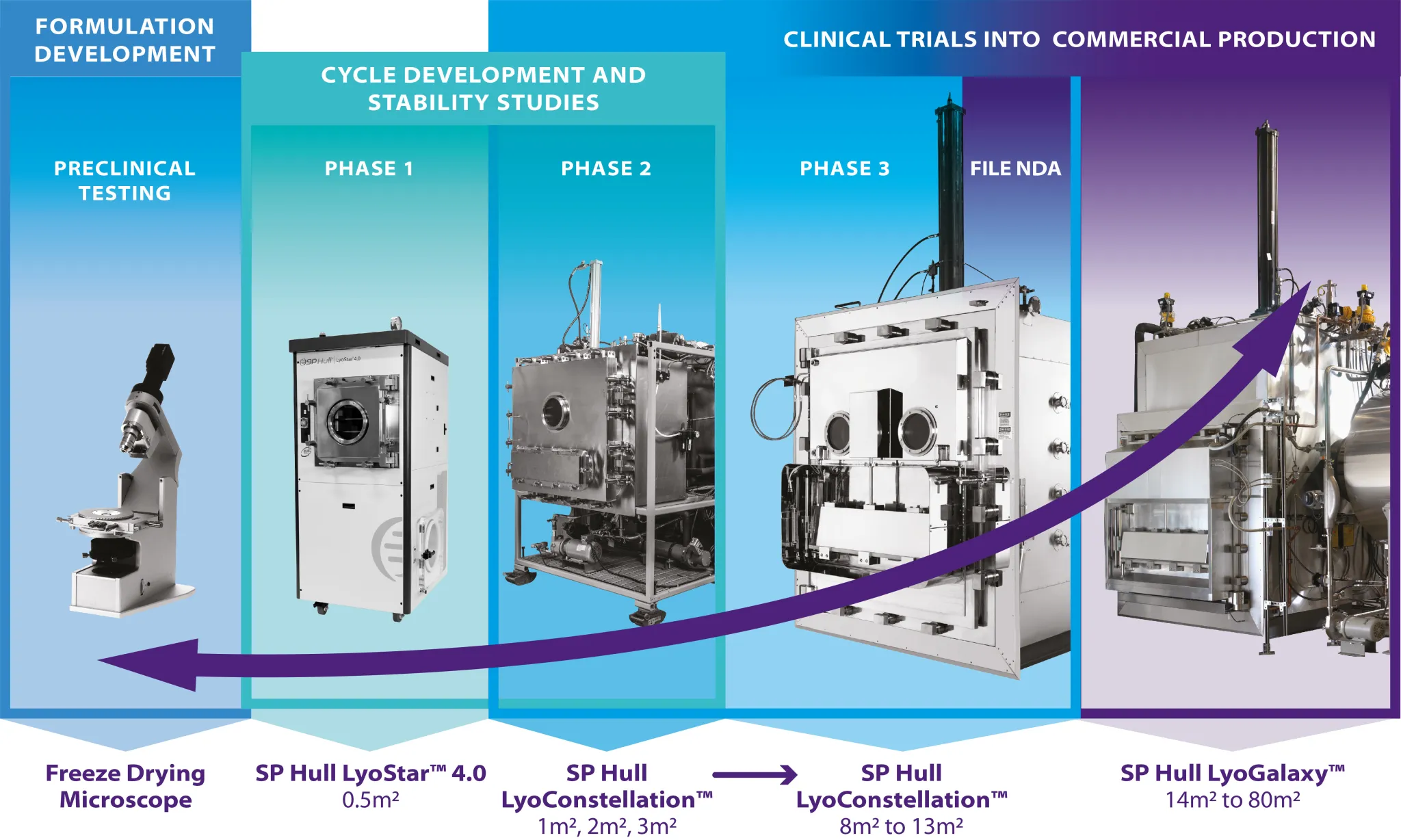
Products Pharmaceutical Processing Equipment Fill-Finish / Aseptic Processing Equipment Aseptic & Production-Scale Freeze Dryers Lyophilization Technology & PAT Tools
-
Applications
Find Products by Applications & Industries
- Brands
-
Learning Lab
Explore the Learning Lab
- Service & Support
-
About Us
Learn more about SP