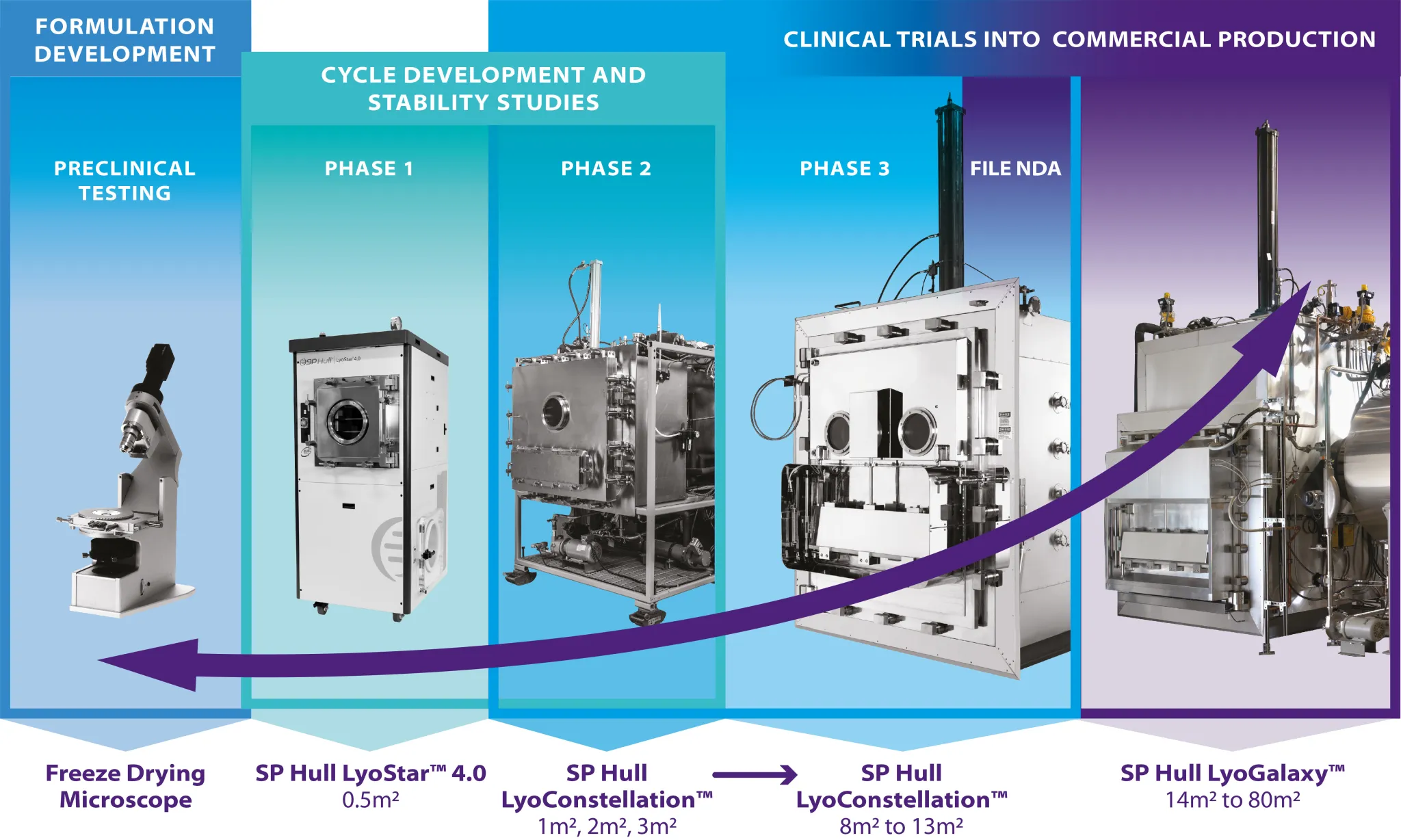
Small batches of lyophilized products can be prepared aseptically and maintain their sterility while using a non-sterilizable freeze-dryer. This webinar will discuss the techniques for preparing the sterile products, containment systems, container/closure systems, the specialized techniques used for lyophilization cycle design, media fill considerations, and some of the Regulatory aspects for this type of operation.
J. Jeff Schwegman, Ph.D. is currently the founder and chief executive officer of Labyrinth BioPharma where he develops formulations, lyophilization cycles, and manufacturing processes for injectable drug products, diagnostics, and tissues for surgery. Additionally, Dr. Schwegman specializes in speaking and consulting in parenteral pre-formulation, formulation, analytical, and lyophilization of both small and large biomolecules. He also holds patents and develops new technologies within the lyophilization field. Dr. Schwegman received his BS in Biochemistry from Indiana University in 1992 and began working at Cook Imaging in Bloomington Indiana, where he gained experience in analytical, formulation, and process development. In 1999 he began graduate study in the Department of Industrial and Physical Pharmacy at Purdue University under the direction of Dr. Steve Nail, where his focus of research involved studying changes in the physical structure of biological molecules during lyophilization. Dr. Schwegman received his PhD from Purdue University in 2003 and returned to Bloomington where he worked at Baxter Pharmaceutical Solutions as a Research Scientist in the Pharmaceutical Development group. He has founded several different companies including BioConvergence and AB BioTechnologies.
Speaker

Jeff Schwegman, PhD
Founder & CEO, Labyrinth BioPharma